-
PDF
- Split View
-
Views
-
Cite
Cite
Tali Lior, Suat Chin Ng, Michael K Ng, A rare case of linitis plastica of the colon from ovarian carcinoma, Journal of Surgical Case Reports, Volume 2019, Issue 3, March 2019, rjz089, https://doi.org/10.1093/jscr/rjz089
Close - Share Icon Share
Abstract
Linitis plastica refers to the diffuse submucosal spread of scirrhous carcinoma in a hollow organ, resulting in constriction and inelasticity of the organ. It is best known to occur in the stomach. Linitis plastica of the colon is rare, and more commonly occurs in the rectum and sigmoid colon from metastatic gastric or breast cancer. We report a case of linitis plastica of the ascending colon in a 69-year-old female as the initial presentation of ovarian carcinoma. Luminal narrowing, poor distension, and rigidity at colonoscopy, in the absence of other causes, should alert the clinician to the potential for an underlying submucosal process, and prompt biopsy techniques that enable submucosal tissue sampling.
INTRODUCTION
Linitis plastica refers to the diffuse infiltration of scirrhous carcinoma within the submucosal and muscularis propria layers of a hollow organ, resulting in constriction, inelasticity and rigidity of the organ [1–3]. Better known to occur in the stomach, linitis plastica of the colon is rare, and usually due to metastatic spread from gastric or breast carcinoma [4]. Diagnosis of linitis plastica of the colon is often delayed due to insidious submucosal spread and mucosal sparing [5]. Strategies that enable higher sensitivity of diagnosis at colonoscopy have not been well defined.
CASE REPORT
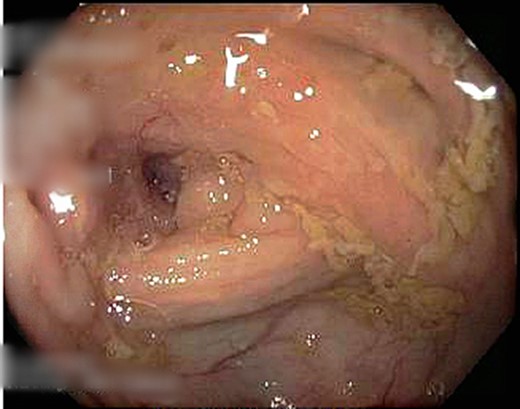
A 69-year-old otherwise well female with no prior history of malignancy underwent colonoscopy for investigation of upper abdominal pain, altered bowel habits—looser, more frequent stools—and subacute weight loss of 8 kg over a number of years. At colonoscopy, luminal narrowing, the impression of colonic thickening and very poor distension was encountered at the ascending colon (Fig. 1). The colonoscope was unable to traverse the narrowed segment. The remainder of the colon was unremarkable and there was no evidence of diverticulosis. Given the normal appearance of the mucosa at the area of presumed pathology, deep biopsies were taken using a bite-on-bite technique in order to ensure that submucosa was captured in the sample. Upper endoscopy was also performed and was normal.
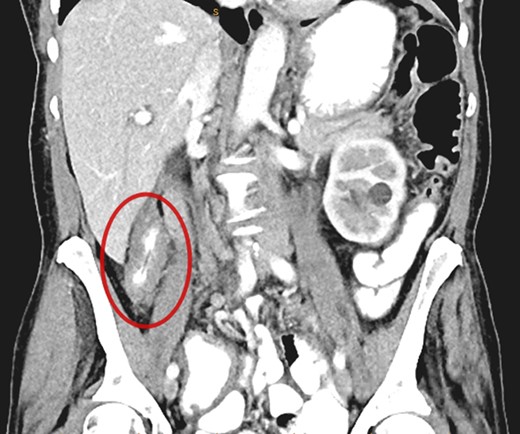
A CT abdomen/pelvis performed on the same day demonstrated contiguous bowel wall thickening from terminal ileum extending to distal ascending colon. There was no evidence of disease elsewhere; notably, there was no evidence of peritoneal disease or ascites.
Submucosal biopsies from ascending colon unexpectedly demonstrated metastatic carcinoma consistent with female genital tract origin—CK7 positive; CK20 negative; PAX-8/WT-1/ER/CA125 all positive. A PET/CT was performed on the basis of the histological findings, which demonstrated intense FDG-avidity in the known site of disease in the ascending colon (Figs 2 and 3), as well as a separate focus of increased metabolism in the left pelvic side-wall (Fig. 4), which was presumed to represent an ovarian primary. There was no evidence of nodal or peritoneal disease, however, note was made of mild bilateral hydroureter without clear transition point. CA125 was 118 U/mL (Ref. range 0–35).
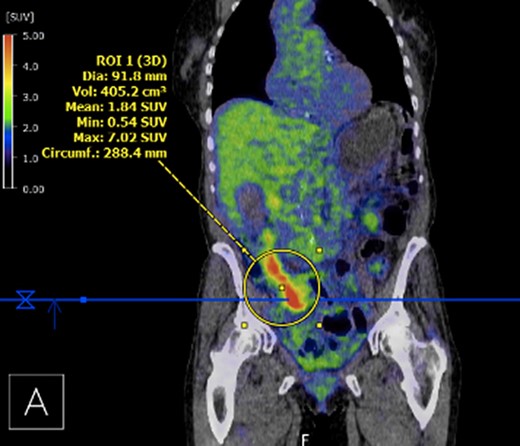
PET demonstrating intense metabolic uptake in thickened ascending colon.
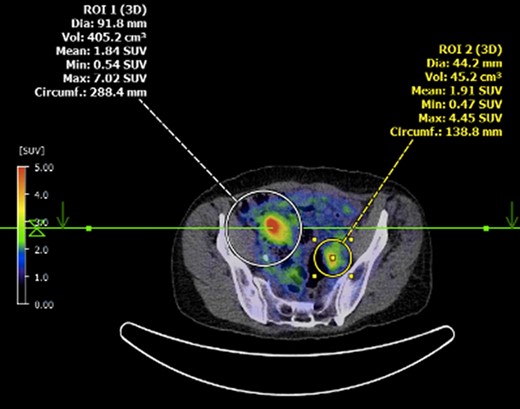
Axial PET showing separate focus of avidity in left pelvic side-wall.
Whilst being worked-up for upfront cytoreductive surgery, the patient’s renal function significantly deteriorated. A repeat CT demonstrated significant progression of disease, with bilateral hydroureter, presumed to be due to retroperitoneal nodal disease, as well as new ascites and peritoneal thickening. The obstructive renal failure was managed with bilateral ureteric stents. Given disease progression and evidence of peritoneal involvement, the recommendation from the multidisciplinary gynae-oncology meeting was for the patient to proceed with neoadjuvant platinum and taxane-based chemotherapy with a plan for restaging and possible cytoreductive surgery thereafter.
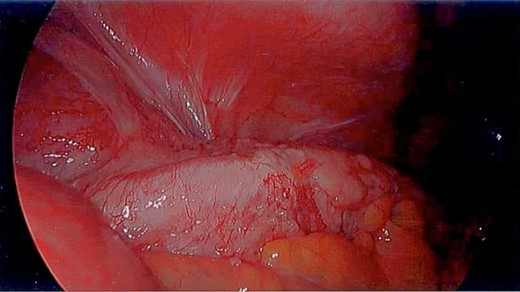
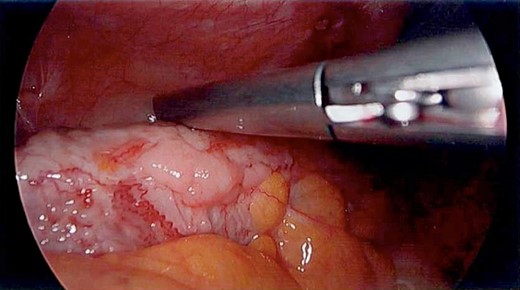
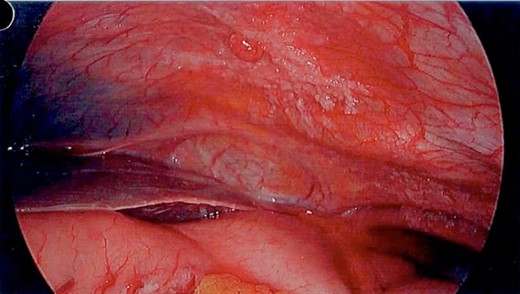
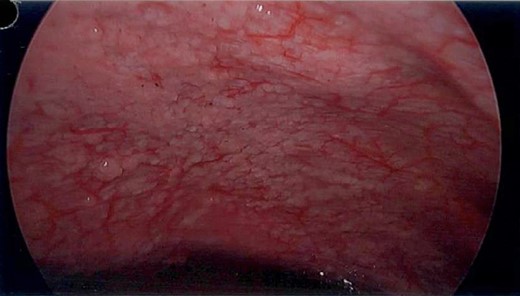
Unfortunately, the patient presented with a large bowel obstruction two weeks after starting chemotherapy. At laparotomy, the point of obstruction was found at the proximal ascending colon, with macroscopic evidence of circumferential mural thickening with a white lathered appearance of the entire right colon (Figs 5 and 6). Peritoneal and patchy omental disease were also observed (Figs 7 and 8)—an omental biopsy was taken which later confirmed an ovarian primary. The decision was made to proceed with a relatively low-risk procedure given that the patient was actively immunosuppressed and had become quite frail; she was therefore defunctioned with a loop ileostomy. The priority was for the patient to be able to continue her neoadjuvant chemotherapy from a survival benefit perspective; the risks of delaying treatment by performing a more extensive resection with its attendant potential complications was not deemed to be in her best interests. A Foley catheter was passed beyond the ileo-caecal valve for decompression. The patient had an uncomplicated recovery postoperatively and was able to complete the scheduled 3-month course of chemotherapy.
Restaging CT after completion of neoadjuvant treatment demonstrated near-complete radiological response to chemotherapy, with mild residual omental haziness without a discrete lesion; ascites had resolved with no evidence of peritoneal disease, nor obvious colonic or small bowel thickening seen. Repeat CA125 was 30 U/mL. The patient is currently awaiting cytoreductive surgery.
DISCUSSION
Linitis plastica of the colon has been reported as a secondary manifestation of gastric carcinoma and lobular breast carcinoma [6], but to our knowledge this represents the only reported case of ovarian carcinoma causing linitis plastica of the colon. Ovarian cancer is notorious for the non-specific spectrum of symptomatology that patients often present with, making early diagnosis a challenge. As this case highlights, signs of disease may be confined to the subtleties identified at colonoscopy.
Linitis plastica is the morphological manifestation of scirrhous carcinoma, and diagnosis is reliant on clinical findings at endoscopy [3]. Diagnosis is often delayed due to its insidious submucosal spread and mucosal sparing, and requires a high index of suspicion at endoscopy. Clinicians must be vigilant for poor inflation, luminal narrowing, and rigidity caused by diffuse mural infiltration [7]. These endoscopic findings are not specific, and may be seen especially in the left colon when there is a history of recurrent diverticulitis and resultant muscle hypertrophy; the absence of diverticuli, and right-sided predominance in this patient gave cause for a higher index of suspicion.
In cases of linitis plastica conventional bite biopsy is unlikely to achieve a diagnosis, given an often-normal overlying mucosa; techniques aimed at submucosal sampling must be employed [8]. Some described techniques, employed in the more common gastric linitis plastica, include bite-on-bite biopsy, strip-and-bite biopsy, as well as EUS-guided FNA [5, 7]. EUS has also been employed in order to establish disruption to mural architecture of the colon [3]. Where biopsies are negative, consideration may be given to further investigation with CT to look for a primary tumour as well as establishing evidence of metastatic disease.
The white leathery appearance of large bowel is a rare finding, and has not been described in relation to linitis plastica of the colon, but if observed in a diagnostic laparoscopy should prompt further investigation as it may represent underlying diffuse disease and potential for large bowel obstruction.
This case highlights the importance of clinician vigilance during colonoscopy in the identification of linitis plastica of the colon. Given its rarity, a high index of suspicion for the subtle colonoscopic findings, as well as knowledge of biopsy techniques that enable submucosal sampling, is required to enable prompt diagnosis and treatment.
CONFLICT OF INTEREST STATEMENT
None declared.



