-
PDF
- Split View
-
Views
-
Cite
Cite
Sudha Pandit, Hrishikesh Samant, Kapil Kohli, Hosein M Shokouh-Amiri, Gregory Wellman, Gazi B Zibari, Incidental liver metastasis in pancreatic adenocarcinoma, Journal of Surgical Case Reports, Volume 2019, Issue 3, March 2019, rjz084, https://doi.org/10.1093/jscr/rjz084
Close - Share Icon Share
Abstract
Exocrine cancer of pancreas is the fourth leading cause of death in the USA among both men and women. Contrast enhanced multidetector-row computer tomography (MDCT) is the current modality of choice for the detection of distant metastasis in pancreatic cancer as a part of pre-operative workup, which helps decide on resectability. Authors present a first ever reported case of an incidental liver metastasis found on intra-operative wedge hepatic biopsy during Whipple’s procedure for pancreatic cancer. This pancreatic cancer was initially thought to be resectable based on MDCT staging per guidelines. The case highlights the importance of diagnostic staging laparoscopy and neoadjuvant chemotherapy before resecting pancreatic adenocarcinoma.
INTRODUCTION
In the USA, approximately 50 000 patients are diagnosed with cancer of the exocrine pancreas annually, and almost all are expected to die from the disease. Surgical resection is the only potentially curative treatment. Unfortunately, because of the late presentation of the disease, only 15–20% of patients are candidates for pancreatectomy. Absolute contraindications to resection include the presence of metastases in the liver, peritoneum, any extra-abdominal site or vascular invasion. Preoperative imaging evaluation determines candidacy for resection. Abdominal CT provides an assessment of local and regional disease extent, which determines resectability, and also evaluates the possibility of distant metastatic spread. Hepatic metastases seen on MDCT or Positron emission tomogram (PET) /CT is a defining characteristic of unresectability based on the consensus-based guidelines from the National Comprehensive Cancer Network (NCCN) [1]. Authors report a case here showing need for better diagnostic tests for management of pancreatic cancer and reason to use of neoadjuvant chemotherapy before curative pancreatic cancer resection.
CASE REPORT
The patient is a 73-year-old elderly Caucasian woman who was initially evaluated for generalized pruritus and painless jaundice for several weeks. Past medical history was significant for Hypertension, and Obesity. Family history and social history was noncontributory. Review of systems was negative except for jaundice and pruritus. Initial laboratory workup revealed WBC 12.0 × 103/ul, Hemoglobin 12.0 g/dl, Platelets 191 × 103/ul. Liver chemistry significant for aspartate aminotransferase (AST) 104 U/l, alanine aminotransferase (ALT) 98 U/l, alkaline phosphatase (ALP) 176 U/l, total bilirubin 5.7 mg/dL and albumin 3.9 g/dL. Carbohydrate antigen 19-9 (CA 19-9) was elevated at 278 u/l. Endoscopic retrograde cholangiopancreatography (ERCP) showed distal common bile duct stricture needing a stent placement. Endoscopic ultrasound (EUS) showed 25 mm diameter pancreatic head mass. Fine needle biopsy of pancreatic head mass was suggestive of adenocarcinoma. MDCT of pancreas (Fig. 1) and liver along with PET /CT scan (Figs 2 and 3) were performed for staging, which were negative for distant metastasis. Patient underwent Whipple’s procedure. Surgical pathology was positive for poorly differentiated pancreatic ductal adenocarcinoma. Lymph nodes involving celiac axis and hepatic artery were negative for malignancy, but 6 out of 28 regional lymph nodes came positive for malignancy on pathology report. Intraoperatively liver parenchyma appeared abnormal and intraoperative ultrasound revealed early fibrosis. Random wedge biopsy from lateral segment of the liver was performed to confirm liver parenchymal disease, which interestingly came positive for 2 mm pancreatic adenocarcinoma metastatic lesion amongst fibrotic liver tissue (Fig. 4). Case was discussed in multidisciplinary conference and referred to oncology for chemotherapy. On 8 months follow up, patient maintained good performance status without any recurrence.
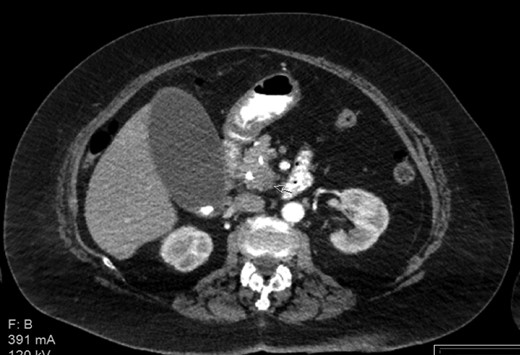
Contrast enhanced CT with pancreatic protocol. Pancreatic head mass.
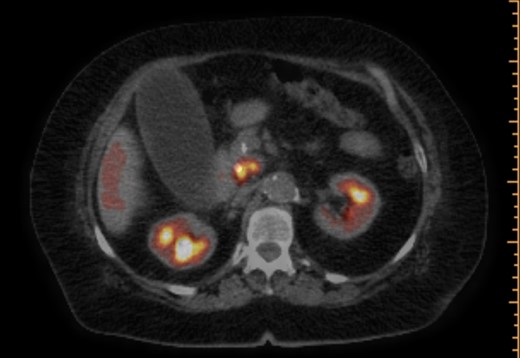
PET/CT scan. Pancreatic head mass with fludeoxyglucose (FDG) uptake.
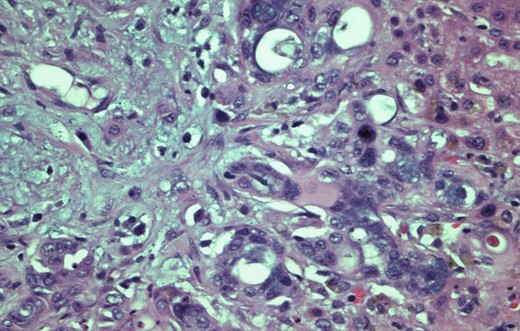
H&E stain, 400× original magnification. Wedge Liver biopsy. Histology diagnostic of pancreatic adenocarcinoma.
DISCUSSION
Cancer of the exocrine pancreas is associated with high mortality. It is the fourth leading cause of cancer-related death in the USA and second only to colorectal cancer as a cause of digestive cancer-related death. The majority of these tumors are adenocarcinomas arising from the ductal epithelium. Contrast-enhanced CT is the modality of choice to detect distant metastases. Triple-phase contrast-enhanced thin-slice (multidetector row) helical computed tomography (MDCT) with three-dimensional reconstruction is the preferred method to diagnose and stage pancreatic cancer. The sensitivity for hepatic metastases is high, particularly using the pancreatic protocol technique. In one study of 43 patients with pancreatic cancer, the sensitivity, specificity, positive predictive value, and negative predictive value of contrast-enhanced multidetector row helical CT for detection of liver metastases were 88, 89, 92 and 84%, respectively [2]. The utility of PET scans in the diagnostic and staging evaluation of suspected pancreatic cancer, particularly whether PET provides information beyond that obtained by contrast-enhanced MDCT, has been controversial. In uncontrolled studies and meta-analyses, the sensitivity of integrated PET/CT (which has better spatial resolution as compared with PET alone) in the initial diagnosis of pancreatic cancer has ranged from 73% to 94%, while specificity ranges from 60% to 89% [3–5]. One possible benefit of PET is enhanced detection of small-volume metastatic disease, which is often missed by CT. Consensus-based guidelines for staging of pancreatic cancer from the NCCN and the European Society for Medical Oncology (ESMO) do not recommend routine use of PET/CT for staging of pancreatic cancer [6].
Currently available imaging techniques are highly accurate at predicting unresectable disease, but they fall short in predicting resectability of disease, mainly because of limited sensitivity for small-volume metastatic disease. Radiographically occult metastases (<1 cm in diameter) on the surface of the liver or peritoneum, which are rarely visible by computed tomography (CT), magnetic resonance imaging (MRI), or transabdominal ultrasound, may be visualized laparoscopically. Diagnostic staging laparoscopy (DSL) has shown to complement the preoperative assessment of radiographic imaging, which has limitations for identifying regional extension of the primary tumor and/or metastatic disease, such as peritoneal involvement [7, 8]. Though in this case liver metastasis was detected incidentally within wedge biopsy of visualized abnormal liver surface and preoperative laparoscopy still could have missed it, authors think that future pancreatic cancer treatment algorithms should include DSL at some point before curative resection and neoadjuvant chemotherapy as it could control these sub-centimeters micro metastasis. There is need for reporting more similar case to understand the impact of sub-centimeter pancreatic metastasis on prognosis of resectable pancreatic cancer.
CONCLUSION
Accurate staging drives proper treatment of patients with pancreatic cancer, particularly when selecting patients for surgical resection. Because most have unresectable disease at presentation, a key goal is to avoid a futile laparotomy whenever possible. This case clearly suggests that MDCT and PET-CT which are part of treatment algorithm for pancreatic cancer still miss distant small metastasis. It is important to note that DSL is cost effective if unresectable disease is identified as the patient is spared of laparotomy, but if performed routinely increases the overall cost of the operative procedure. Authors, by presenting this case, intend to highlight whether DSL should be included in staging workup of certain pancreatic adenocarcinomas and also support the role of neoadjuvant chemotherapy before curative resections to control missed micro metastatic in pancreatic adenocarcinoma.
CONFLICT OF INTEREST STATEMENT
None declared.



