-
PDF
- Split View
-
Views
-
Cite
Cite
John D L Brookes, Manish Mathew, Charlene P Munasinghe, John C Gribbin, David A Devonshire, Prashant Joshi, Andrew D Cochrane, Pseudocyst of the pancreas masquerading as spontaneous pneumomediastinum, Journal of Surgical Case Reports, Volume 2019, Issue 3, March 2019, rjz068, https://doi.org/10.1093/jscr/rjz068
Close - Share Icon Share
Abstract
Pseudocyst of the pancreas extending into the thorax represents a rare but potentially catastrophic diagnosis. It can be difficult to both diagnose and manage, with only limited management suggestions within the literature. While pleural effusion is a common complication of pancreatitis, transthoracic extension of a pseudocyst is a rare phenomenon. Herein we discuss a patient with a difficult to recognize extension of pancreatic pseudocyst into the left hemithorax, with unique imaging findings. He had good response to trans-gastric and percutaneous drainage and ultimately proceeded to thoracotomy and decortication. Around this case, the options for investigation and management are discussed.
INTRODUCTION
Pancreatic pseudocyst (PP) is a collection of inflammatory and pancreatic fluid surrounded by granulation tissue without epithelial lining. Forming in response to pancreatitis, the fluid includes pancreatic enzymes capable of local tissue destruction [1]. Dissecting through tissue planes PP may track into the thorax most commonly via the Oesophageal or Aortic Hiatuses, and rarely through Morgagni’s Foramen or Traumatic tracts. The literature contains only limited management suggestions, without standardized guidelines [2, 3]. Herein we discuss our approach to a massive pancreatic pseudocyst extending into the left Hemithorax.
CASE
A 40-year-old gentleman was transferred to our tertiary-centre with acute pancreatitis post-endoscopic biopsy of the Ampulla of Vater.
His only past history was diverticulitis, with no previous abdominal surgeries, he was a non-smoker, with minimal alcohol consumption. Following a recent episode of diverticulitis he underwent Colonoscopy and Gastroscopy at which the ampulla looked grossly abnormal and was biopsied to exclude malignancy (histopathology was unremarkable). He subsequently developed severe abdominal pain and Lipase 25 000 U/l. External CT showed acute pancreatitis with no bowel perforation.
He was managed with restricted diet, naso-gastric drainage and aggressive analgesia. He progressed well, diet was upgraded, pain well controlled and repeat CT noted only a small left pleural effusion and changes consistent with known pancreatitis. He was discharged home.
Two days later he represented with severe back pain and vomiting, tachycardic and febrile. CT Scan showed: Pancreatitis, no evidence of necrosis, and a small-to-moderate pleural effusion. After initial conservative management on interval monitoring he developed necrosis and multiple peripancreatic fluid collections consistent with pseudocysts. In light of ongoing pain, tachycardia and tachypnea in keeping with a septic picture he was moved to Intensive Care. Antibiotics were broadened and he proceeded to EUS-guided Cystogastrostomy, draining one litre of purulent fluid. The fluid was too viscous to assess Lipase.
Chest X-ray in ICU following drainage showed what was thought to be Pneumomediastinum (Figs 1 and 2), and given this concern he was referred to the Cardiothoracic service. Subsequent CT suggested a large loculated hydro-pneumothorax, which in retrospect represented the drained Pseudocyst communicating through the diaphragm (Figs 3 and 4).
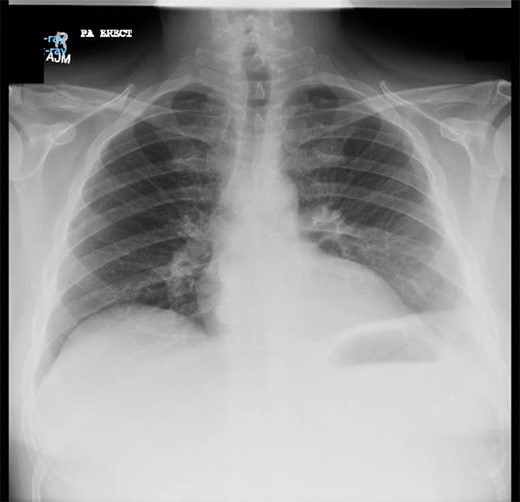
Chest X-ray prior to transgastric drainage. Suggestive of Left pleural effusion/ lower lobe collapse.
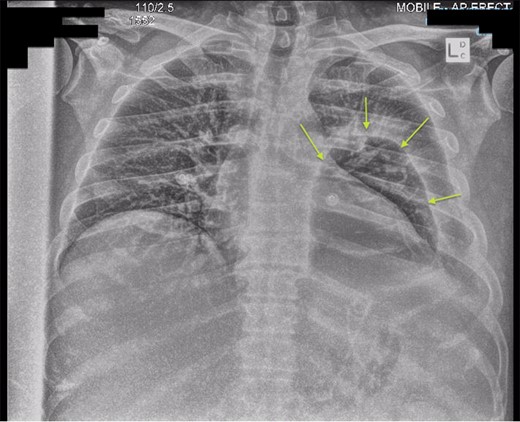
Chest X-ray post-transgastric drainage. Area of residual space highlighted with arrows.
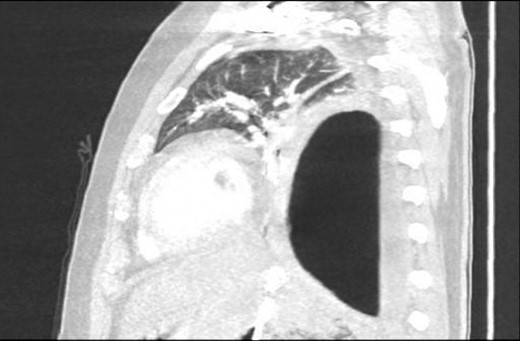
Sagittal view CT Chest revealing large residual space with loss of left lung volume due to the pseudocyst. Image post-transgastric drainage.
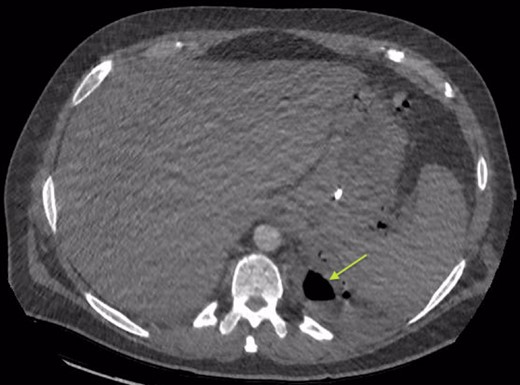
Area of communication between the drained Pseudocyst and the Left Pleura. Space noted with arrow.
An Intercostal drain was inserted under CT-guidance aiming to decompress the pleural space. Amylase level in the pleural drainage was 880 U/l, Lipase 472 U/l. There was significant reduction of the Hydropneumothorax (Fig. 5). Given his young age, potential for loss of lung volume and infection he proceeded to decortication.
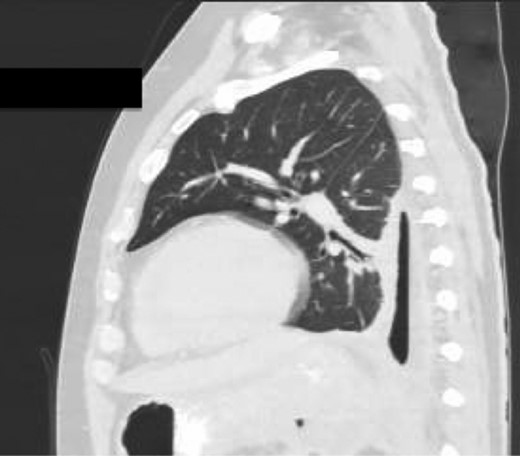
Sagittal view CT Chest post-intercostal catheter drainage of the Pancreatico-pleural fistula. Smaller residual space can be noted compared to Image 3, with some ongoing loss of lung volume and small fluid collection.
The operation was performed via mini-thoracotomy, finding an infected cavity, loculated fluid with pus flakes. No abnormal tract through the diaphragm was noted. The thick pleural rind was separated and the lung released with complete re-expansion. He was transferred to the ward with two drains. These were removed day four when the small air-leak resolved. Subsequent thoracic imaging showed complete re-expansion (Fig. 6).
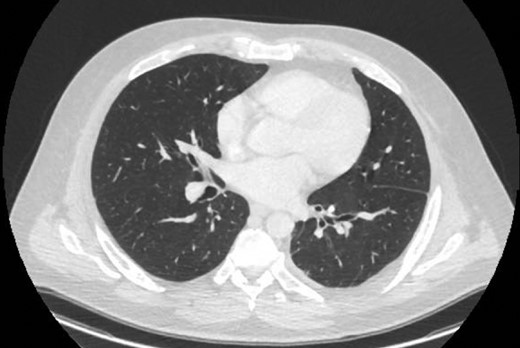
CT one month post-decortication with complete re-expansion of the left lung and minor post-operative changes noted posteriorly.
He later underwent cholecystectomy revealing chronic cholecystitis. His course was prolonged by superior mesenteric vein and splenic vein thrombus, likely secondary to pseudocyst compression, which was treated with clexane.
DISCUSSION
PP occurs in approximately 2% of patients with pancreatitis. However only 0.4% have intra-thoracic extension [4]. About 80–90% are due to alcoholic pancreatitis [5]. Thoracic extension of pancreatic pseudocysts (TPP) is a rare entity with as few as 75 cases reported. However, it is worth noting the severe implications with 29–39% of deaths from pancreatitis attributable to pulmonary complications [2]. If not recognized TPP may have disastrous complications, including: pneumonia, empyema, tamponade, pseudoaneurysm, oesophageal perforation, massive haemoptysis and Bronchopleural fistula [1, 6, 7].
Patients may present with a myriad of symptoms. TPP predominantly results in thoracic symptoms; dyspnoea (65%), cough (27%), chest pain (23%) and dysphagia [5].
Prompt diagnosis of TPP is vital due to the potentially life-threatening complications. The diagnosis of TPP can be confirmed by Amylase in pleural drainage >5 000 U/l. This patient’s Amylase was likely lower due to previous transgastric drainage and pleural infection. Radiologically CT, ERCP and MRCP are most common, with sensitivity to diagnose TPP 47%, 78% and 80%, respectively [8]. As highlighted by our case it can be difficult to differentiate the body of the pseudocyst from a reactive pleural phenomena, ours only becoming evident post-transgastric drainage, by the appearance of air in the thoracic cavity which must have been in continuity with the abdominal disease.
The literature suggests first-line management should involve less invasive measures and surgery be reserved for those not responding to therapy. However, medical management of TPP has a resolution rate of 30–60% with 15% recurrence, and 12% mortality. In contrast, operative management has a success rate of up to 90% with 18% recurrence [9]. Initial medical management includes; Octreotide, total parenteral nutrition, and bromhexine hydrochloride—all aimed at decreasing pancreatic secretions. Radiologically-guided drainage may be undertaken targeting the intra-thoracic or intra-abdominal pseudocyst. Otherwise cystogastrostomy, Duodenoscope and Needle-knife papillotome may be performed as minimally invasive procedures. More invasive procedures such as Pancreatic head resection, Distal pancreatectomy, or from the thoracic approach decortication or resection with pedicled intercostal muscle flap to close the fistula through the diaphragm have also been described (though tendency for diaphragmatic breaches to close following aspiration of fluid is noted) [3, 10].
Ajmera proposed one of the only algorithms for management of TPP [3]. They recommended unstable patients with life-threatening complications proceed to surgery, including patients with TPP complicated by rupture, infection or major haemorrhage. Management of stable patients depends upon the size of the pseudocyst and symptoms. Symptomatic patients or those with large TPP should be treated with drainage (preferably endoscopic). Asymptomatic patients could be managed conservatively with careful clinical and radiological follow-up [3]. Our case outlines the potential for using transgastric and transthoracic drainage as an effective temporizing measure. More extensive surgery was then performed to achieve definitive source-control of infection and preserve the patient’s long-term respiratory function.
CONCLUSION
TPP represents a rare but potentially catastrophic diagnosis. TPP can be difficult to diagnose and multiple imaging modalities are often required. We describe a case of an unstable patient, with difficult to recognize TPP and unique imaging findings, who responded well to trans-gastric and percutaneous drainage. However with view to long-term function and risk of infection he proceeded to thoracotomy and decortication. An excellent post-operative outcome was achieved.
CONFLICT OF INTEREST STATEMENT
No conflict of interest.
The patient provided written informed consent for publication of case and use of anonymized images.
FUNDING
The authors received no financial support for the research, authorship and publication of this article.



