-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher J Micallef, Jamie N Johnson, R Michael Johnson, A value analysis of microsurgical lower extremity reconstruction vs. acellular urinary bladder matrix (UBM) for radiation wounds of the lower extremity, Journal of Surgical Case Reports, Volume 2019, Issue 3, March 2019, rjz051, https://doi.org/10.1093/jscr/rjz051
Close - Share Icon Share
Abstract
In the USA, external beam radiation is offered to patients as an alternative to surgery for non-melanoma skin cancers. While this technique may be useful in highly specific patient populations, recalcitrant chronic radiation wounds can result. These complex wounds ultimately may require major reconstructive surgery to achieve closure. Porcine urinary bladder matrix (UBM) may be effective in the treatment of radiation wounds and eliminating the need for vascularized tissue transfers. A case report of an elderly male with bilateral radiation wounds of the lower extremity, one extremity treated with free flap reconstruction and the other with porcine urinary bladder matrix, is presented.
INTRODUCTION
Skin cancer is the most common cancer affecting patients in the USA and external beam radiation is offered to certain patients as an alternative to surgery for non-melanoma skin cancers. Unfortunately, the immediate and long-term effects of external beam radiation therapy may result in significant morbidity, including chronic ulceration, pain, secondary infection, and fibrosis [1]. Reconstruction of radiated wounds frequently requires flap reconstruction to bring vascularized tissue into the damaged area. Tissue-engineered skin substitutes have shown the potential to heal complex wounds [2, 3]. The primary advantage of these products is to reduce the donor site morbidity and associated cost of a tissue transfer. Urinary bladder matrix (UBM) may be a useful new modality in the treatment of diabetic foot ulcers, venous stasis ulcers, pressure ulcers, and radiation wounds [4].
The case report presented is a patient with bilateral lower extremity radiation wounds. One side required treatment with free tissue transfer and skin graft and the other extremity was healed with porcine UBM, avoiding the need for a second free tissue transfer.
CASE REPORT
A 77-year-old otherwise healthy, active male smoker with squamous cell carcinoma of the bilateral lower extremities was treated with primary external beam radiation at another healthcare facility. In addition to local recurrence after treatment, the radiation therapy resulted in non-healing ulcers on both legs—exposed tibia and tibialis anterior tendon on the right and a chronic indolent medial mid-calf wound on the left. Initially, the patient was treated bilaterally with local wound care, surgical debridement and hyperbaric oxygen. In 2010, a free latissimus dorsi muscle flap and skin graft were performed that resulted in successful healing of the right leg (Fig. 1). However, on the left lower extremity, chronic wounds persisted (Fig. 2).
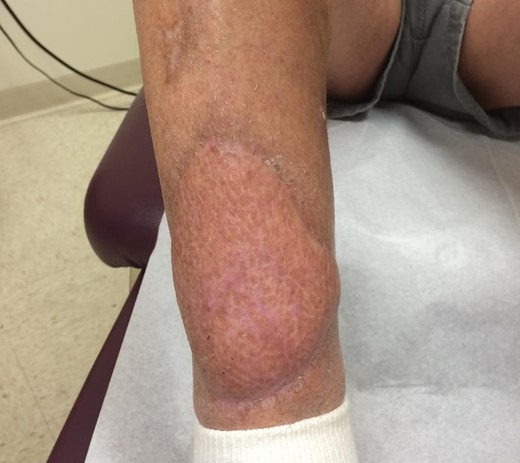
Right lower extremity with fully healed free latissimus dorsi flap coverage of previous radiation wounds.
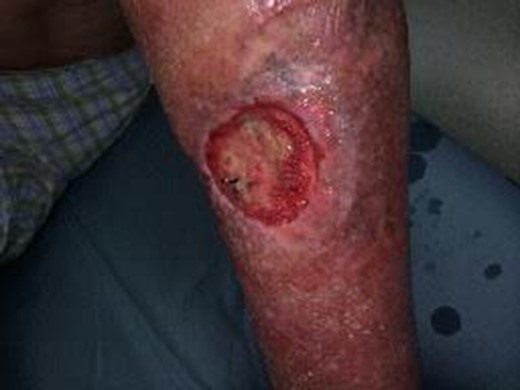
Left lower extremity with persistent non-healing radiation wound after multiple attempts at bilateral lower extremity wound care and hyperbaric treatment. Wound depicted is prior to initiation of UBM therapy.
The wounds on the left leg were further managed conservatively as there was no bone exposure. Biopsy of the chronic wound confirmed no residual cancer. Over the next two years, conservative treatment with wet to dry dressings, alginate silver, and bilaminate dermal regeneration template (Integra® Bilayer Wound Matrix, Integra LifeSciences Corp, Plainsboro NJ) was attempted without success. While the patient was satisfied with the free flap reconstruction of the right limb, he was extremely active and did not wish to undergo another large surgical reconstruction on the left limb. A final attempt of conservative treatment with UBM was initiated before proceeding to another free tissue transfer to the left leg. The patient was followed weekly in the clinical office where UBM powder and sheets (MatriStem® Wound Matrix system, now marketed as Micromatrix® and Cytal® respectively, ACell, Inc., Columbia MD) were re-applied until complete wound closure and re-epithelialization had occurred. Dressings included xeroform®, bacitracin ointment, and rolled gauze bandage. Through this process, our patient achieved complete healing of the lower extremity radiation wound without infection or complication within a treatment time of approximately seven weeks (Fig. 3).
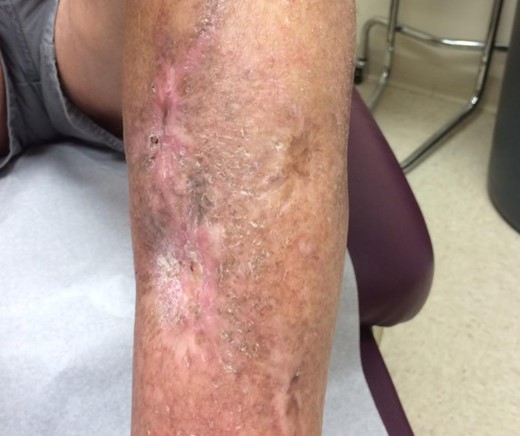
Left lower extremity six months after completion of UBM therapy with healed wound.
Accurate financial comparisons are somewhat challenging due to a lack of transparency in the US healthcare system. However, after evaluating the financial records, the hospital charges for the free latissimus dorsi flap totaled $76 300. The surgeon charge was $10 000, with $2079 being reimbursed on that charge. Over the course of treatment with UBM, the total reimbursement for office visits was approximately $567. UBM is categorized in the low-cost biologic product category and the cost of the product was $2428 for all treatments. Current procedural terminology (CPT) codes now exist for application of skin substitutes, but reimbursement is not uniform and, when used in the hospital, is frequently bundled into diagnosis related group (DRG) payments.
DISCUSSION
The precise mechanism of action of UBM in radiation wounds is unclear. There is no evidence that the direct DNA damage caused by radiation is reversed. More likely, as proposed by Brown et al, the scaffolding effect allows migration of adjacent healthy keratinocytes and possibly influences M2 regenerative macrophages, both of which are enhanced by porcine UBM [5].
Review of the literature demonstrates few clinical in vivo studies evaluating the effect of UBM on complex wound healing. One retrospective study by Lechaminant et al. showed full healing of 34 wounds (including diabetic, venous, ischemic, and decubitus) with reduced healing time from an average of 25.5 weeks to a mean of 9.8 weeks following initial application of UBM after failed closure with local wound care. In this study, patients had stable wounds at follow up from 9 months to 3 years [6]. Another small study by Rommer et al, also showed complete wound healing of recalcitrant radiation wounds after UBM application with continued closure noted at follow-up from 18-24 months [7]. The patient in this report also experienced long term (over 4 years) stable wound closure with UBM treatment of his radiation wounds.
The obvious limitation of this study is the anecdotal nature of a case report. Additionally, healing cannot be absolutely determined as solely due to the UBM applications. However, the temporal nature of the biologic application would suggest UBM to be a significant contributor to the healing process. Although, it is possible prior treatments ‘primed’ the wound for closure with UBM. In the authors’ experience, UBM is not universally successful in the treatment of difficult wounds, especially if there is significant tendon or bone exposure.
As health care costs continue to be scrutinized, optimization of outcomes has become more crucial. The use of porcine UBM in this patient eliminated the additional co-morbidities and costs associated with undergoing a second free flap. Despite this, while the product is FDA approved, it is difficult to obtain reimbursement due to limited data on outcomes. Recently, Porter published a value equation that argues that value is equivalent to the outcomes that matter to patients divided by the cost [8]. Ultimately, the patient was extremely satisfied and relieved not to undergo an additional complex surgery. Additionally, the cost of the continued attempts at local wound care is likely much less than invasive surgical procedures. However, due to the lack of transparency in medical billing, this cannot be definitively shown. Therefore, there is the significant potential for UBM to provide good value to patients, even after other modalities of conservative therapies have failed to provide definitive closure of the wound.
CONFLICT OF INTEREST STATEMENT
None declared.



