-
PDF
- Split View
-
Views
-
Cite
Cite
Rebecca Cutts, Martin J Connor, Luxi Sun, Thomas Johnston, Rachel Gooch, John McLoughlin, Mesothelioma subtypes of the tunica vaginalis: a rare case report and review of histological criteria, Journal of Surgical Case Reports, Volume 2019, Issue 3, March 2019, rjz040, https://doi.org/10.1093/jscr/rjz040
Close - Share Icon Share
Abstract
Well-differentiated papillary mesothelioma (WDPM) is a rare histological subtype of mesothelioma arising from the tunica vaginalis. We present a case of a 23-year-old male with a palpable para-testicular lump of 3 years duration. Scrotal exploration revealed a grossly abnormal cystic appearance of his tunica vaginalis. An excision biopsy confirmed WDPM of the tunica vaginalis. The three subtypes of mesothelial tumours of the tunica vaginalis are described by their distinct histological features, tumour growth and reported prognosis. A summary of immunohistochemistry and the surgical management across the disease spectrum is provided. Recent clarification of the histological criteria of WDPM provides the opportunity for surgeons to offer a limited approach to managing this indolent tumour that mimics malignant mesothelioma. However, the lack of evidence on recurrence and progression rates in WDPM restricts most surgeons to performing a radical orchidectomy, as was performed in this case.
INTRODUCTION
Mesotheliomas arise from transformation of mesothelial cells that line the pleura, pericardium and peritoneum. Rarely, mesotheliomas originate from the serous tunica vaginalis. Three distinct subtypes exist: (1) well-differentiated papillary mesothelioma (WDPM), (2) mesothelioma of uncertain malignant potential (MUMP) and (3) malignant mesothelioma (MM). WDPM is a rare, histological mimic of malignant mesothelioma that has an indolent course, but no agreed urological management.
CASE REPORT
A 23-year-old male presented with an intermittently painful left scrotal swelling, which had gradually enlarged over a 3-year period. There was no history of scrotal trauma, exposure to asbestos or family history of mesothelioma.
He was initially treated empirically for epididymo-orchitis. An ultrasound scan revealed a 4 cm cystic structure with a small amount of internal echogenic debris and a mildly thickened wall with evidence of vascularity. There was also prominence of the left scrotal veins. Scrotal exploration revealed a grossly abnormal cystic appearance of his tunica vaginalis and an excision biopsy was performed.
Histological features of the specimen were that of a fibrous cyst wall covered by cuboidal cells with bland nuclei (Fig. 1). Within the cystic space, there were branching, papillary structures with a fibrovascular core, lined with a single layer of bland cuboidal cells (Fig. 2). The stroma was oedematous and hyalinised. No sub-epithelial invasion was seen. There was no unequivocal cytologic atypia or atypical mitosis.
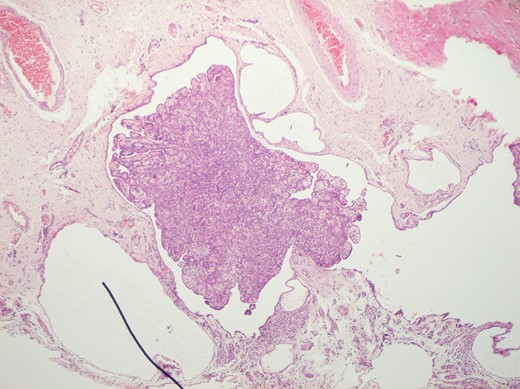
H&E staining of the specimen demonstrating a fibrous cyst wall covered by cuboidal cells with bland nuclei.
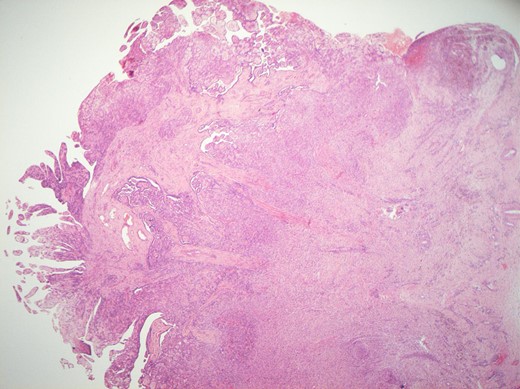
H&E staining of the specimen demonstrating branching, papillary structures with a fibrovascular core lined with a single layer of bland cuboidal cells.
Immunohistochemistry performed reported the cuboidal cells were positive for EMA and focally positive for Calretinin (a specific marker for cells of mesothelial origin). P53 staining was positive and MIB-1 (Ki-67) stains were present on <5% of the cells. The overall morphology and immune-profile are suggestive of a well-differentiated papillary mesothelioma.
Following a multi-disciplinary team discussion, a radical orchidectomy was performed without complication. Specimen pathology confirmed clear excision margins, presence of WDPM and no features of MM. A post-operative CT scan of the abdomen and pelvis confirmed no extra-testicular disease. At 18 months post-orchidectomy the patient had no features of diseases recurrence.
DISCUSSION
Well-differentiated papillary mesothelioma of the tunica vaginalis was first described by Barbara and Romino in 1957 [1]. WDPM is the least commonly encountered subtype, but importantly is thought to be a benign and indolent tumour with a favourable prognosis. Therefore, the morphological criteria for WDPM are extremely important, but remains controversial [2].
Brimo et al. proposed that the WDPM definition should be restricted to tumours that show exclusive papillary architecture in which the papillae are lined by a single layer of bland cuboidal cells, as in this reported case [2]. For safety, those tumours that do not meet this definition ought to be labelled as mesothelioma of uncertain malignant potential or malignant mesothelioma and treated accordingly [2, 3]. A comparison of histological findings, using this definition, is presented in Table 1.
Summary of morphology, tumour growth, and prognosis in mesothelioma subtypes.
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Papillary morphology | Present | Predominates | Focal Areas |
| Complex morphology | None | Focal areas | Present |
| Mitotic Activity | None | None or very few | Present |
| Atypical Mitosis | None | None | Present |
| Cellular Atypia | None | None | Present |
| Coagulation Necrosis | None | None or micro-focal | Present |
| Stromal Invasion | None | None | Present |
| Psammoma Bodies | None | None | Present |
| Tumour Growth | Indolent | Indolent with uncertain malignant potential | Aggressive neoplasm |
| Prognosis | Good | Unclear, malignant potential | Poor |
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Papillary morphology | Present | Predominates | Focal Areas |
| Complex morphology | None | Focal areas | Present |
| Mitotic Activity | None | None or very few | Present |
| Atypical Mitosis | None | None | Present |
| Cellular Atypia | None | None | Present |
| Coagulation Necrosis | None | None or micro-focal | Present |
| Stromal Invasion | None | None | Present |
| Psammoma Bodies | None | None | Present |
| Tumour Growth | Indolent | Indolent with uncertain malignant potential | Aggressive neoplasm |
| Prognosis | Good | Unclear, malignant potential | Poor |
WDPM, well-differentiated papillary; MUMP, mesothelioma of uncertain malignant potential; MM, malignant mesothelioma.
Summary of morphology, tumour growth, and prognosis in mesothelioma subtypes.
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Papillary morphology | Present | Predominates | Focal Areas |
| Complex morphology | None | Focal areas | Present |
| Mitotic Activity | None | None or very few | Present |
| Atypical Mitosis | None | None | Present |
| Cellular Atypia | None | None | Present |
| Coagulation Necrosis | None | None or micro-focal | Present |
| Stromal Invasion | None | None | Present |
| Psammoma Bodies | None | None | Present |
| Tumour Growth | Indolent | Indolent with uncertain malignant potential | Aggressive neoplasm |
| Prognosis | Good | Unclear, malignant potential | Poor |
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Papillary morphology | Present | Predominates | Focal Areas |
| Complex morphology | None | Focal areas | Present |
| Mitotic Activity | None | None or very few | Present |
| Atypical Mitosis | None | None | Present |
| Cellular Atypia | None | None | Present |
| Coagulation Necrosis | None | None or micro-focal | Present |
| Stromal Invasion | None | None | Present |
| Psammoma Bodies | None | None | Present |
| Tumour Growth | Indolent | Indolent with uncertain malignant potential | Aggressive neoplasm |
| Prognosis | Good | Unclear, malignant potential | Poor |
WDPM, well-differentiated papillary; MUMP, mesothelioma of uncertain malignant potential; MM, malignant mesothelioma.
Following this work a recent systematic review applied the updated morphological criteria of WDPM to 24 previously published cases and concluded that only 8 cases demonstrated histological of ‘true’ WDPM [3].
Immunohistochemistry of subtypes of mesothelioma of the tunica vaginalis is poorly reported (Table 2). While WDPM and MM can be differentiated histologically, the immunohistochemistry similarities limit role to a diagnostic adjunct. Hai et al. reported calretenin to be the most sensitive (100%) and specific (91.3%) marker of mesothelioma [4]. There are no reported cases of WDPM without positive calretenin (Table 2) and it remains consistently reported in cases of MM [5]. The p53 tumour marker, reported in this case, is documented in two other cases of WDPM with an increased 58% sensitivity and 91% specificity in MM [2, 3, 6]. Epithelial membrane antigen (EMA) remains intermittently reported in both WDPM and MM [6–8] we cannot identify positive reports of CEA and Leu M-1 antibody in either subtype (Table 2).
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Calretenin | Consistently reported | Consistently reported | Consistently reported |
| CK 5/6 | Intermittently reported | Intermittently reported | Intermittently reported |
| Epithelial membrane antigen (EMA) | Intermittently reported | Intermittently reported | Intermittently reported |
| Carcinoembryonic antigen (CEA) | No reported cases | No reported cases | No reported cases |
| Leu M – 1 ab | No reported cases | No reported cases | No reported cases |
| p53 | Intermittently reported | Intermittently reported | Intermittently reported |
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Calretenin | Consistently reported | Consistently reported | Consistently reported |
| CK 5/6 | Intermittently reported | Intermittently reported | Intermittently reported |
| Epithelial membrane antigen (EMA) | Intermittently reported | Intermittently reported | Intermittently reported |
| Carcinoembryonic antigen (CEA) | No reported cases | No reported cases | No reported cases |
| Leu M – 1 ab | No reported cases | No reported cases | No reported cases |
| p53 | Intermittently reported | Intermittently reported | Intermittently reported |
Intermittently reported—≤5 reported cases, consistently reported—>5 reported cases.
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Calretenin | Consistently reported | Consistently reported | Consistently reported |
| CK 5/6 | Intermittently reported | Intermittently reported | Intermittently reported |
| Epithelial membrane antigen (EMA) | Intermittently reported | Intermittently reported | Intermittently reported |
| Carcinoembryonic antigen (CEA) | No reported cases | No reported cases | No reported cases |
| Leu M – 1 ab | No reported cases | No reported cases | No reported cases |
| p53 | Intermittently reported | Intermittently reported | Intermittently reported |
| . | WDPM . | MUMP . | MM . |
|---|---|---|---|
| Calretenin | Consistently reported | Consistently reported | Consistently reported |
| CK 5/6 | Intermittently reported | Intermittently reported | Intermittently reported |
| Epithelial membrane antigen (EMA) | Intermittently reported | Intermittently reported | Intermittently reported |
| Carcinoembryonic antigen (CEA) | No reported cases | No reported cases | No reported cases |
| Leu M – 1 ab | No reported cases | No reported cases | No reported cases |
| p53 | Intermittently reported | Intermittently reported | Intermittently reported |
Intermittently reported—≤5 reported cases, consistently reported—>5 reported cases.
Clinically, WDPM of the tunica vaginalis presents a significant diagnostic challenge, occurring in a wide range of age groups with few identifiable risk factors beyond the presence of a hydrocele (absent in this case). Inflammation, trauma and infection have also been theorized as potentially involved in the development of papillary proliferative lesions [2]. MM of the tunica vaginalis presents in a similar fashion, with approximately 50% presenting as hydroceles, which may delay definitive diagnosis [2, 3]. For the operating urologist, concerning macroscopic features suggestive of MM of the tunica vaginalis include thickening, multi-focal nodules and ‘friable papillary fronds’ [9].
There is no gold standard treatment for WDPM of the tunica vaginalis [3]. Radical orchidectomy is the most common approach as in this case. Although, in younger men where access to cryosection (frozen section) is available, some advocate fertility sparing surgery in the form of wide local excision [3, 9]. Two reported cases have been managed with such an approach, although the long-term oncological outcomes are unknown [3, 10]. In contrast, MUMP and MM dictate a radical orchiectomy, with or without hemi-scrotectomy and retroperitoneal lymph node dissection [9].
The prognosis of WDPM is good with the WHO classing the tumour as benign [2]. However, MUMP has an unclear prognosis with reports of malignant transformation [2, 3]. Malignant mesothelioma is however, an aggressive tumour, with a median survival of 23 months [2, 9].
The histological clarification of WDPM provides the opportunity for surgeons to offer conservative approaches to managing this indolent tumour that mimics malignant mesothelioma. However, the lack of evidence on recurrence and progression rates in WDPM limits most surgeons to performing a radical approach. Any change in operative approach in these men would need to be supported with access to intra-operative cryosection and evidence on favourable long-term oncological outcomes.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
PATIENT CONSENT
Gained.



