-
PDF
- Split View
-
Views
-
Cite
Cite
Shigeo Ninomiya, Kazuaki Hiroishi, Akio Shiromizu, Yoshitake Ueda, Norio Shiraishi, Masafumi Inomata, Tsuyoshi Arita, Gastrointestinal stromal tumor of the lesser omentum: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2019, Issue 2, February 2019, rjz035, https://doi.org/10.1093/jscr/rjz035
Close - Share Icon Share
Abstract
A 72-year-old woman visited our hospital for a routine health examination and underwent abdominal ultrasonography, which revealed an intra-abdominal tumor. Abdominal computed tomography and magnetic resonance imaging showed a well-defined solid mass of ~3 cm in diameter lying adjacent to the stomach. The mass was preoperatively diagnosed as gastrointestinal stromal tumor of the stomach. At laparotomy, a well-encapsulated tumor was found in the lesser omentum. It was slightly adherent to the stomach wall but was removed without difficulty. Therefore, only enucleation of the tumor was performed. The excised tumor, which was 35 × 30 × 25 mm3 in size, had a white cut surface without necrosis or hemorrhage. According to the pathological findings, the tumor was classified as a very low-risk gastrointestinal stromal tumor originating in the lesser omentum. Gastrointestinal stromal tumor of the lesser omentum is very rare, and surgical resection is the only effective treatment modality.
INTRODUCTION
Since Hirota et al. [1] first described a mesenchymal tumor of the stomach with a c-KIT mutation, gastrointestinal stromal tumors (GISTs) have become the most commonly found mesenchymal neoplasm arising from the gastrointestinal tract. The stomach and small intestine are the most common locations; GISTs are rarely located in the colon, rectum or esophagus. However, extra-GISTs (EGISTs) are extremely rare. They are usually located in the omentum or in the mesentery and account for 5–10% of all GISTs [2]. In addition, to our best knowledge, only nine cases of GISTs of the lesser omentum have been reported previously. We report a case of GIST of the lesser omentum and review the literature.
CASE REPORT
A 72-year-old Japanese woman visited our hospital for a routine health examination and underwent abdominal ultrasonography, which revealed an intra-abdominal tumor. The patient had no symptoms, and the findings of physical and laboratory tests, including carcinoembryonic antigen and carbohydrate antigen 19-9 were unremarkable. The patient had no history of previous illness and was not taking any regular medications. Contrast-enhanced computed tomography (CT) (Fig. 1a) and magnetic resonance imaging (MRI) (Fig. 1b) showed a well-defined, mildly heterogeneous solid mass of about 3 cm in diameter lying adjacent to the stomach. Endoscopy showed the lesser curvature of the stomach to be compressed extraluminally and without mucosal abnormalities (Fig. 2). An endoscopic ultrasonogram revealed a 30 × 25-mm2 low-echoic lesion that was adhered to the side of the lesser curvature of the stomach. Based on these findings, the mass was preoperatively diagnosed as a GIST of the stomach.
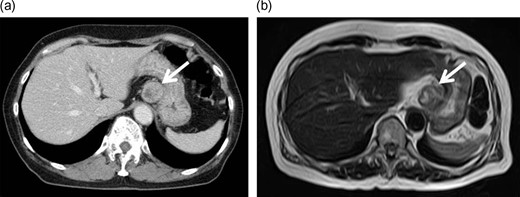
Enhanced computed tomography (CT) and magnetic resonance imaging (MRI). Contrast-enhanced CT (a) and MRI (b) images showed a well-defined solid mass of ~3 cm in diameter lying adjacent to the stomach, with some heterogeneity.
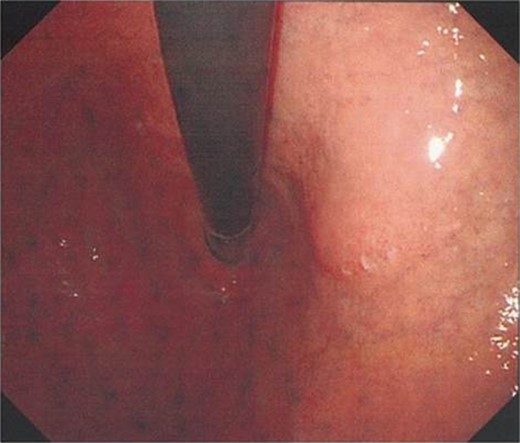
Upper endoscopy findings. Upper endoscopy showed the lessor curvature of the stomach to be compressed extraluminally without mucosal abnormalities.
At laparotomy, a well-encapsulated tumor was found in the lesser omentum. It was slightly adhered to the stomach wall but could be removed without difficulty (Fig. 3). There were no obvious lesions of peritoneal dissemination or lymph node metastasis. Therefore, only enucleation of the tumor was performed. The excised tumor, which was 35 × 30 × 25 mm3 in size, had a white cut surface without necrosis or hemorrhage (Fig. 4). Histologically, the tumor was composed of spindle-shaped cells with an interlacing bundle pattern exhibiting microcystic changes and moderate cellularity (Fig. 5a). No mitotic figures were observed. Immunohistochemistry revealed the tumor cells to be positive for c-KIT (CD117) (Fig. 5b), myeloid stem cell antigen (CD34) (Fig. 5c) and α-smooth muscle antigen (α-SMA) (Fig. 5d) and negative for S-100 protein (Fig. 5e). Based on Fletcher’s classification, the tumor was classified as a low-risk GIST originating in the lesser omentum. The patient had an uneventful postoperative course. Presently, the patient is alive, without recurrence, and is doing well 3 years after the surgery without imatinib treatment.
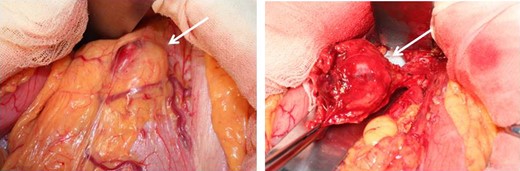
Operative findings. At laparotomy, a well-encapsulated tumor was found in the lesser omentum. It was slightly adhered to the stomach wall but was removed without difficulty.
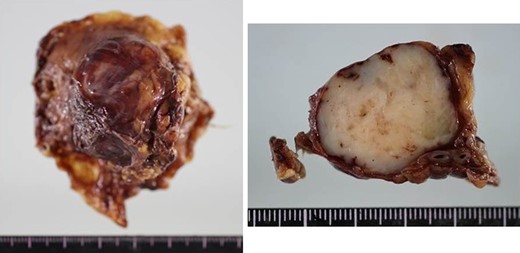
Macroscopic findings of the resected specimens. The excised tumor, which was 35 × 30 × 25 mm3 in size, had a white cut surface without necrosis or hemorrhage.
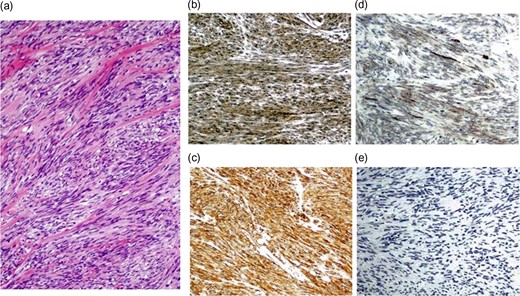
Pathological findings of the resected specimens. Histologically, the tumor was composed of spindle-sharped cells (a). Immunohistochemistry revealed the tumor cells to be positive for c-KIT (b), CD34 (c) and α-SMA (d) and negative for S-100 protein (e).
DISCUSSION
EGISTs comprise only ~5–10% of all GISTs [2]. Approximately 80% of EGISTs are located in the omentum or mesentery, and GISTs of the lesser omentum are extremely rare. To our best knowledge, only nine cases of GISTs of the lesser omentum have been reported previously (Table 1). As shown in Table 1, the tumor arose in five men and five women ranging in age from 22 to 79 years (median 69 years). The tumor diameters ranged from 27 to 210 mm (median 110 mm). Histologically, they were classified as spindle cell type (n = 8) or epithelioid cell type (n = 2). However, the clinicopathological features of GISTs of the lesser omentum have not been fully elucidated because of their rarity. Therefore, further accumulation of cases is necessary.
Reported cases of gastrointestinal stromal tumor of the lesser omentum (including our case).
| Study . | Age (years) . | Sex . | Preoperative diagnosis . | Size (mm) . | Cell type . | Recurrence . |
|---|---|---|---|---|---|---|
| Takahashi et al. [3] | 71 | F | Tumor of the lesser curvature | 170 | Spindle | None |
| Fukuda et al. [4] | 45 | M | Extramucosal tumor of the stomach | 45 | Spindle | None |
| Sakurai et al. [8] | 74 | F | Unknown | 115 | Spindle | Unknown |
| Fagkrezos et al. [2] | 63 | M | Unknown | 160 | Spindle | None |
| Nakaya et al. [9] | 69 | M | No operation | 210 | Spindle | NA |
| Aihara et al. [5] | 22 | M | GIST of the stomach | 27 | Spindle | Unknown |
| Ogawa et al. [6] | 69 | M | Hepatic hemangioma | 80 | Epithelioid | None |
| Skandalos et al. [10] | 79 | F | Tumor of the pancreas | 110 | Spindle | None |
| Trombatore et al. [7] | 69 | F | Hepatic hemangioma | 110 | Epithelioid | None |
| Present case | 72 | F | GIST of the stomach | 35 | Spindle | None |
| Study . | Age (years) . | Sex . | Preoperative diagnosis . | Size (mm) . | Cell type . | Recurrence . |
|---|---|---|---|---|---|---|
| Takahashi et al. [3] | 71 | F | Tumor of the lesser curvature | 170 | Spindle | None |
| Fukuda et al. [4] | 45 | M | Extramucosal tumor of the stomach | 45 | Spindle | None |
| Sakurai et al. [8] | 74 | F | Unknown | 115 | Spindle | Unknown |
| Fagkrezos et al. [2] | 63 | M | Unknown | 160 | Spindle | None |
| Nakaya et al. [9] | 69 | M | No operation | 210 | Spindle | NA |
| Aihara et al. [5] | 22 | M | GIST of the stomach | 27 | Spindle | Unknown |
| Ogawa et al. [6] | 69 | M | Hepatic hemangioma | 80 | Epithelioid | None |
| Skandalos et al. [10] | 79 | F | Tumor of the pancreas | 110 | Spindle | None |
| Trombatore et al. [7] | 69 | F | Hepatic hemangioma | 110 | Epithelioid | None |
| Present case | 72 | F | GIST of the stomach | 35 | Spindle | None |
NA = not applicable; GIST = gastrointestinal stromal tumor.
Reported cases of gastrointestinal stromal tumor of the lesser omentum (including our case).
| Study . | Age (years) . | Sex . | Preoperative diagnosis . | Size (mm) . | Cell type . | Recurrence . |
|---|---|---|---|---|---|---|
| Takahashi et al. [3] | 71 | F | Tumor of the lesser curvature | 170 | Spindle | None |
| Fukuda et al. [4] | 45 | M | Extramucosal tumor of the stomach | 45 | Spindle | None |
| Sakurai et al. [8] | 74 | F | Unknown | 115 | Spindle | Unknown |
| Fagkrezos et al. [2] | 63 | M | Unknown | 160 | Spindle | None |
| Nakaya et al. [9] | 69 | M | No operation | 210 | Spindle | NA |
| Aihara et al. [5] | 22 | M | GIST of the stomach | 27 | Spindle | Unknown |
| Ogawa et al. [6] | 69 | M | Hepatic hemangioma | 80 | Epithelioid | None |
| Skandalos et al. [10] | 79 | F | Tumor of the pancreas | 110 | Spindle | None |
| Trombatore et al. [7] | 69 | F | Hepatic hemangioma | 110 | Epithelioid | None |
| Present case | 72 | F | GIST of the stomach | 35 | Spindle | None |
| Study . | Age (years) . | Sex . | Preoperative diagnosis . | Size (mm) . | Cell type . | Recurrence . |
|---|---|---|---|---|---|---|
| Takahashi et al. [3] | 71 | F | Tumor of the lesser curvature | 170 | Spindle | None |
| Fukuda et al. [4] | 45 | M | Extramucosal tumor of the stomach | 45 | Spindle | None |
| Sakurai et al. [8] | 74 | F | Unknown | 115 | Spindle | Unknown |
| Fagkrezos et al. [2] | 63 | M | Unknown | 160 | Spindle | None |
| Nakaya et al. [9] | 69 | M | No operation | 210 | Spindle | NA |
| Aihara et al. [5] | 22 | M | GIST of the stomach | 27 | Spindle | Unknown |
| Ogawa et al. [6] | 69 | M | Hepatic hemangioma | 80 | Epithelioid | None |
| Skandalos et al. [10] | 79 | F | Tumor of the pancreas | 110 | Spindle | None |
| Trombatore et al. [7] | 69 | F | Hepatic hemangioma | 110 | Epithelioid | None |
| Present case | 72 | F | GIST of the stomach | 35 | Spindle | None |
NA = not applicable; GIST = gastrointestinal stromal tumor.
Accurate preoperative diagnosis of EGISTs is difficult. Despite the use of advanced radiological imaging techniques, it is difficult to differentiate a GIST in the lesser omentum from that in the lesser curvature of the stomach. As Table 1 shows, four cases of GIST of the lesser omentum, including our case, were misdiagnosed as extramucosal tumors of the stomach [3–5]. Additionally, two cases of GIST of the lesser omentum were followed up as a diagnosis of benign hepatic tumor without surgery [6, 7]. Trombatore et al. [7] reported a case with rapid growth on CT and MRI after a 16-month follow-up period. Therefore, we should also include GIST of the lesser omentum in the differential diagnosis of abdominal masses when the lesion shows features that are uncommon for GIST, such as a location in the lesser omentum but outside the gastrointestinal tract.
GISTs are thought to be potentially malignant tumors, and their malignant potential has been assessed using various parameters, such as tumor size and the number of mitotic figures, necrosis, cell type and Ki-67 labeling index. The most reliable and simple criteria for distinguishing malignant GISTs are a tumor of ≥5 cm in size with a mitotic rate of 5/50 high-power fields or more. According to previous criteria, the present case was diagnosed as a low-grade malignancy. However, some authors reported that tumor size is not a reliable prognostic factor in the case of GISTs of the lesser omentum [2, 6]. In contrast, Aihara et al. [5] reported that GISTs of the lesser omentum were considered to have lower malignant potential than GISTs originating from the gastrointestinal tract. Thus, the malignant potential of GISTs of the lesser omentum has not been clearly established, and careful follow-up will continue to be needed in the future.
The current definitive treatment of GISTs, including EGISTs, is complete surgical resection. An extensive lymphadenectomy is not required because of the low incidence of lymph node metastasis. For our patient, enucleation of the tumor was performed without injury to the pseudocapsule because it could not be surgically detached from the stomach. No postoperative recurrences of GISTs of the lesser omentum have been reported previously (Table 1). Therefore, surgical resection currently appears to be an effective treatment for GISTs of the lesser omentum. In conclusion, GISTs of the lesser omentum are extremely rare, and surgical resection is an effective treatment modality. However, the malignant potential of these tumors needs to be clearly established.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest regarding the publication of this report.
REFERENCES
- magnetic resonance imaging
- hemorrhage
- laparotomy
- necrosis
- abdomen
- neoplasms
- stomach
- gastrointestinal stromal tumor
- cellular enucleation
- abdominal ultrasonography
- abdominal ct
- lesser omentum
- stomach wall
- excision
- dishonesty
- enucleation procedure
- treatment effectiveness
- diameter
- gastric gastrointestinal stromal tumor



