-
PDF
- Split View
-
Views
-
Cite
Cite
Geon Hi Park, Byoung Chul Lee, Dong woo Hyun, Jung Bum Choi, Young Mok Park, Hyuk Jae Jung, Hong Jae Jo, Mechanical intestinal obstruction following laparoscopic inguinal hernia repair in a patient with abdominal cocoon syndrome, Journal of Surgical Case Reports, Volume 2019, Issue 12, December 2019, rjz370, https://doi.org/10.1093/jscr/rjz370
Close - Share Icon Share
Abstract
Abdominal cocoon syndrome (ACS) is a rare condition characterized by partial or complete encasement of small intestine by a thick fibro-collagenous membrane. A 65-year-old man presented to the surgical department with left inguinal. He underwent laparoscopic transabdominal preperitoneal inguinal hernia. When we inserted a trocar into the peritoneal cavity, the small intestine was injured and repaired immediately. We identified a fibrotic membrane covering the small intestine, which was found as ACS. Two weeks later after discharge, he presented to the emergency department with mechanical intestinal obstruction. Conservative treatment had no effect on the patient and membrane excision, adhesiolysis and small intestine resection with anastomosis were performed. Unfortunately, the patient was hospitalized for a long time with bowel leakage and discharged on postoperative day 48. The preoperative diagnosis is quite challenging and most cases are diagnosed intraoperatively. When finding the ACS during the operation, careful attention should be needed.
INTRODUCTION
Sclerosing encapsulating peritonitis is defined as rare condition that refers to partial or total encapsulation of the small intestine by a fibro-collagenous membrane [1]. The idiopathic or primary form termed abdominal cocoon syndrome (ACS) by Foo et al in 1978 [2]. Intestinal obstruction is the main clinical manifestation of ACS. There are many reported cases of intestinal obstruction caused by ACS. ACS is fairly difficult to diagnose intraoperatively in case of lack of characteristic symptoms. We report on a patient who had asymptomatic ACS and received treatment of mechanical intestinal obstruction caused by laparoscopic trocar injury to the small intestine.
CASE REPORT
A 65-year-old man presented to the surgical department with left inguinal hernia found in medical examinations and he had no significant medical history. Laparoscopic transabdominal preperitoneal inguinal hernia was performed. When we inserted a trocar into the peritoneal cavity, the trocar directly entered the bowel because of the presence of adhesions. We then repaired the injured small intestine and identified a fibrotic membrane covering the small intestine. He was diagnosed with ACS. Hernia repair was completed by converting to laparoscopic totally extraperitoneal repair with mesh. On the third postoperative day he was discharged without complications.
Two weeks later after discharge, he presented to the emergency department with abdominal pain, nausea, vomiting and inability to defecate or pass gas. On examination he was afebrile and his vitals were stable. Only distension of the abdomen was observed. The laboratory examinations were normal, except high CRP level (4.27 mg/dL). Plain abdominal X-rays showed air–fluid levels and abdominal CT showed adhesions at the periumbilical area with dilated small bowel (Fig. 1). He was initially managed conservatively with bowel rest, nasogastric decompression and nutritional support.
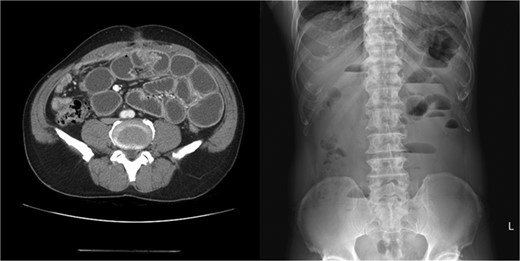
Abdomen CT and X-ray showed adhesions at the periumbilical area with dilated small bowel.
Conservative treatment had no effect on the patient and exploratory laparotomy was performed. On exploration, there were a dense fibrous sac encasing the entire small intestine and dense adhesions between intestinal loops. We performed excision of the membrane off the intestinal surface, complete enterolysis with release of the bowel loops, and resection and anastomosis of small bowel that caused obstruction (Figs. 2 and 3).
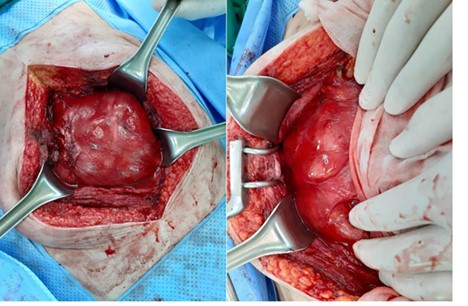
Whole small intestine is encapsulated in a dense fibrous membrane.
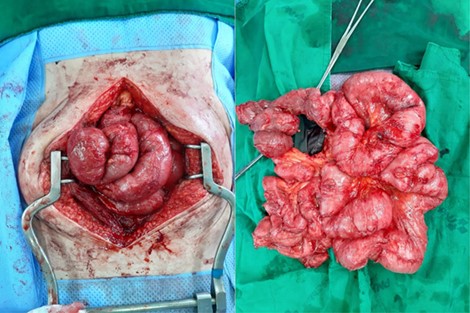
After excision of the membrane off the intestinal surface and complete enterolysis.
The patient manifested clinical signs of ileus on postoperative day 10 and was managed conservatively. Postoperative day 14, he complained of abdominal pain and fever, after which greenish discharge drained through the operation wound. He was suspected of bowel leakage and urgently taken into operation. On laparotomy, there was 200–300 ml greenish fluid in the peritoneal cavity and peritoneal lavage was done. We could not explore the leakage site because of the very severe adhesion after surgery. Multiple drainage tube was inserted under suspicion of anastomotic leakage. We planned to treat the patient conservatively with abdominal drainage, nasogastric decompression, antibiotics, and nutritional support. After 4 weeks of conservative treatment, he recovered well and started diet. Then, he recovered gradually and was discharged on postoperative day 48. He is being followed up without any symptoms for 6 months.
The Institutional Review Board of Pusan National University Hospital approved this study and waived the informed consent requirement.
DISCUSSION
The term primary SEP, secondary SEP, ACS and peritoneal encapsulation (PE) are erroneously used in many previous articles on SEP [3]. PE is a developmental anomaly characterized by the congenital presence of an accessory peritoneal membrane. SEP is an acquired condition resulting from peritoneal inflammation that may be triggered by various factors [4]. Depending on the underlying causes, SEP divided into primary (idiopathic) and secondary forms. The idiopathic form was named as ACS [2]. Akbulut et al. [3] reported that there were several case studies including 193 patients with ACS. Their ages ranged from 7 to 87 years and 122 (66.3%) patients were male. Of the 149 patients, 65 (43.6%) underwent operations for a presumed diagnosis on ACS.
While some cases have an asymptomatic course, most of ACS are generally acute or subacute intestinal obstruction. Among patients presenting to emergency department with intestinal obstruction, 60–80% caused by postoperative adhesions, while 6% are caused by unusual causes [2]. ACS is one of the unusual causes leading to intestinal obstruction; hence, clinical suspicion is important in diagnosing ACS. CT is the most helpful imaging modality for the diagnosis of ACS. The entire dilated small bowel at the center of the abdomen and encased within a thick fibro-collagenous membrane as though it were in a cocoon on abdominal CT are diagnostic [5]. However, the preoperative diagnosis of ACS is difficult because ACS is rarely seen and its clinical symptoms are non-specific. In a retrospective study, it was reported that only 16% of the cases could be preoperatively diagnosed [6].
Surgery is the first choice of symptomatic ACS in almost all literatures. Peeling the membrane off the intestinal surface and excising the dense adhesions between the intestinal loops and bowel resection is unnecessary unless there is an ischemic change. Laparoscopy is not part of the standard surgical approach in patients with ACS [3]. There are a limited number of case reports, which have described successful laparoscopic membrane excision and adhesiolysis [7]. In our study, we happened to injure small intestine while we inserted a trocar and there was a similar case in the past [8]. It is thought that laparotomy with adhesiolysis and removal of membrane should have been performed after incidental laparoscopic trocar injury.
The most common postoperative complications are postoperative small bowel obstruction (EPSBO), intra-abdominal infection, enterocutaneous fistula, short bowel syndrome, and bowel perforation [9]. The recurrence of the ACS in the postoperative period is rare, but most probable complication is ESPBO due to adhesions. ESPBO usually develops within 30 days postoperatively in patients who have undergone extensive adhesiolysis and excision and were managed well with bowel rest and total parenteral nutrition. Enterocutaneous fistula and bowel perforation are occurred as a result of secondary damage or anastomosis leakage.
In conclusion, ACS is a rare condition with unknown cause and most cases are still incidentally diagnosed during abdominal surgery. However, careful excision of membrane with release of the small intestine can lead to complete recovery.
FINANCIAL DISCLOSURE
None reported.
CONFLICT OF INTEREST
None declared.
REFERENCES
- anastomosis, surgical
- emergency service, hospital
- intestinal obstruction
- intestine, small
- intestines
- laparoscopy
- tissue membrane
- greater sac of peritoneum
- peritonitis
- preoperative care
- surgical procedures, operative
- diagnosis
- trocar
- laparoscopic inguinal hernia repair
- excision
- adhesiolysis
- conservative treatment
- encapsulation
- properitoneal inguinal hernia
- sclerosing encapsulating peritonitis



