-
PDF
- Split View
-
Views
-
Cite
Cite
Yaying Eileen Xu, Kimberley Tan, Rasika Hendahewa, Intra-abdominal tuberculosis masquerading as ovarian carcinoma, Journal of Surgical Case Reports, Volume 2019, Issue 12, December 2019, rjz361, https://doi.org/10.1093/jscr/rjz361
Close - Share Icon Share
Abstract
Intra-abdominal tuberculosis (TB) is rare in Australia, but it can be found in those who emigrate from endemic areas. We report a rare case of a 28-year-old lady from the Philippines who presented with abdominal pain, ascites and an elevated cancer antigen (CA) 125 with an initial concern of ovarian malignancy. She underwent a diagnostic laparoscopy which revealed typical features of intra-abdominal TB and histological features of granulomatous inflammation. The symptoms and signs of intra-abdominal TB are non-specific and can mimic many other conditions. The gold-standard mode of diagnosis in intra-abdominal TB is laparoscopy with tissue biopsy. Ovarian malignancy is relatively rare in pre-menopausal women; hence an elevated CA 125 warrants a broader differential diagnosis. It is important to have intra-abdominal TB as a differential even in the non-endemic settings to avoid delay in diagnosis and appropriate management.
INTRODUCTION
Tuberculosis (TB) is common in the developing world, and it is one of the top 10 leading causes of death worldwide. TB is rare in Australia; however, it is not uncommon amongst those who are born overseas. Intra-abdominal TB accounts for 1–2% of all TB cases and one of the most common forms of extra-pulmonary TB. The symptoms of intra-abdominal TB are not specific as they can mimic many other diseases such as Crohn’s disease, lymphoma and malignancy [1, 2]. We report a rare case of a pre-menopause lady who presented with abdominal pain, elevated cancer antigen 125 (CA 125) and ascites with an initial concern for a gynaecological malignancy who was later diagnosed with intra-abdominal TB.
CASE REPORT
A 28 -year-old lady from the Philippines presented with a 1-month history of right upper quadrant pain and suprapubic pain, bloating and night sweats. Her background history includes chronic hepatitis B for which she has ongoing follow-up with a liver physician. On examination, she had normal observations; her abdomen was distended with mild tenderness throughout. Her blood tests revealed a normal white cell count and liver functions, and the liver screen was consistent with chronic hepatitis B infection. Her CA125, however, was elevated at 218 units per millilitre (U/mL). The other tumour markers, including carcinoembryonic antigen, carbohydrate antigen 19.9 and alpha fetoprotein, were all normal. An ultrasound of her abdomen showed a 16 mm left para-ovarian cyst, thickened fallopian tubes and moderate free fluid. She subsequently had a computed tomography (CT) scan, which showed ascites with diffuse peritoneal thickening encasing small bowel loops (Fig. 1A and B). The gynaecological team was consulted as there was initial concern of a possible ovarian cancer. She had an ascitic tap, which showed lymphocytic rich fluid, with no evidence of malignancy. The patient also had no previous contacts with TB, and her TB screening 6 years ago for her visa was negative.
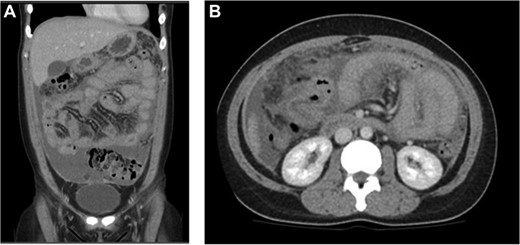
(A) Coronal and (B) axial views: diffuse peritoneal thickening with moderate ascites and encasement of small bowel loops.
She had an urgent diagnostic laparoscopy by the general surgical team, which showed a physiological left ovarian cyst, a number of peritoneal and omental nodules (which were biopsied) and reactive serous fluid with extensive adhesions (Fig. 2A–C). The histology showed granulomatous inflammation; however, the DNA specific for Mycobacterium tuberculosis complex was not detected. She was reviewed by the infectious diseases team who diagnosed her with intra-abdominal TB based on the clinical history, histopathology, and she responded well to anti-TB medications.
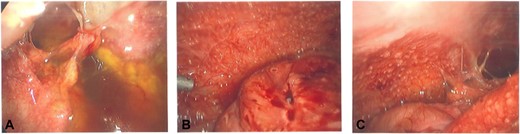
(A–C) intra-operative photos demonstrating reactive ascites, yellow peritoneal nodules, and thickened bowel loops.
DISCUSSION
There are four forms of intra-abdominal TB: peritoneal, nodal, luminal and visceral involving intra-abdominal solid organs. The luminal form of intra-abdominal TB is the most common form especially involving the ileo-caecal junction [3]. It can be spread via the haematogenous route from a lung focus, the lymphatics from infected lymph nodes and ingestion of bacilli from infected food [1]. The manifestations of intra-abdominal TB are non-specific, and they depend on the organ involved. The patient may present with abdominal pain, diarrhoea, bleeding from the gastrointestinal tract, weight loss, night sweats, fever and abdominal distention with ascites [4].
The diagnosis of intra-abdominal TB is challenging. There are several criteria in diagnosing intra-abdominal TB: (i) positive acid fast bacilli (AFB) smear or culture from peritoneal fluid, (ii) histological features of caseating granulomas and (iii) typical presentation and response to anti-TB medications [1]. The AFB test from ascitic fluid is easy to obtain; however, it often has a low positive rate. The polymerase chain reaction of blood or ascitic fluid is unable to differentiate between living and dead bacteria; hence, it remains positive for a long period even after completion of anti-TB treatment [5]. Both the ultrasound and CT are able to detect ascites, thickened bowel wall and lymphadenopathy; however, these features can mimic many other diseases. The colonoscopy is especially helpful in diagnosing intestinal TB. Laparoscopy with tissue biopsy is the gold-standard modality in diagnosing intra-abdominal TB [6, 7].
There is generally a good response of intra-abdominal TB to medications which is a combination of rifampicin, isoniazid, pyrazinamide and ethambutol for 6 months. Cho et al. (2018) looked at the outcome of 139 patients over 12 years in Korea with intra-abdominal TB; 84.2% of patients showed a complete response to anti-TB treatment, with a 1.4% mortality rate; 54.7% of their patients had microbiological or histological evidence of TB; in the remaining 45.3%, there was a clinical diagnosis [1].
In another study, Bevin et al. (2018) performed a retrospective study in Christchurch, which identified 20 patients diagnosed with intra-abdominal TB over 20 years, and they found all patients (10 patients out of the 20 patients) who underwent laparoscopy had positive biopsies. The ascitic fluid was obtained from nine patients, which all had negative AFB results; however, 3 of 9 had mycobacterial growth from the culture [6]. It demonstrated that laparoscopy with tissue biopsy is the gold-standard for diagnosing peritoneal TB, typical features include yellow/white nodules scattered over the peritoneum; omental thickening with ascites; and an abdominal cocoon with matted small bowel [8]. The patient in this case report had most of the features of intra-abdominal TB.
The CA 125, also known as mucin 16, is found in the respiratory tract and the female reproductive tract epithelia. It can be elevated in many benign and malignant conditions, such as endometriosis, heart failure, lymphoma and ovarian cancer. It is also known to be elevated in intra-abdominal TB, which makes ovarian malignancy a common differential when woman presents with an elevated level [5]. The clinical features are important in differentiating TB from malignancy in non-endemic countries. Also, women with intra-abdominal TB tended to be younger than women with malignancy [2].
The diagnosis of intra-abdominal TB remains difficult due to its non-specific features. This case highlights the importance of intra-abdominal TB being a differential for non-specific abdominal pain and ascites, particularly in young person born overseas; with greater awareness, there may be less delay to diagnosis and treatment.



