-
PDF
- Split View
-
Views
-
Cite
Cite
Nirvana B Saraswat, William B DeVoe, Metastatic melanoma of the gallbladder presenting as polyp in acute cholecystitis, Journal of Surgical Case Reports, Volume 2019, Issue 12, December 2019, rjz324, https://doi.org/10.1093/jscr/rjz324
Close - Share Icon Share
Abstract
Malignant melanoma is an aggressive neural crest cell-derived neoplasm with a propensity for metastasis to almost any organ. Gastrointestinal metastasis may manifest as gallbladder polyps. We report a case of metastatic malignant melanoma diagnosed in an 81-year-old male after cholecystectomy performed for acute cholecystitis. Cholecystectomy remains the standard of care for treatment of isolated gallbladder metastasis, especially in the setting of symptomatic disease. Mutation-directed chemotherapeutic and immunotherapeutic modalities serve as efficacious adjunctive therapy in addition to primary surgical resection for this rare condition.
INTRODUCTION
Malignant melanoma is a rare albeit aggressive neoplasm. Arising from melanocytes of neural crest origin, melanoma is most commonly associated with exposure to sunlight, specifically ultraviolet B radiation. It is the fifth most common cancer among men and women in the USA [1]; the worldwide incidence has steadily increased in the past half-century, a trend thought secondary to increased radiation exposure as a result of factors such as ozone depletion and artificial radiation [2, 3].
Melanoma exhibits a propensity for metastasis to the lung, liver, soft tissue and skin. Less than 5% metastasize to the gastrointestinal tract, of these, the majority (67%) involve the small intestine. The gallbladder is an uncommon site of metastasis. In contrast, the most common origin of metastatic gallbladder tumors is melanoma [1]. Melanoma has been reported both de novo and as a metastatic focus in the gallbladder, largely in the form of individual cases after Weiting and Hamdi’s sentinel report in 1907 [4]. Wang et al. described an exceptionally rare form of primary gallbladder melanoma metastatic to duodenum, adrenal gland and celiac lymph node [5]. Primary gallbladder melanoma is a diagnosis of exclusion when no prior diagnosis of melanoma nor any potential primary sites identified, as pathologic analysis cannot absolutely substantiate the origin of neoplastic melanoma tissue. Metastatic melanoma has been reported masquerading as an asymptomatic polyp, a solid lesion, emphysematous cholecystitis without discrete lesions, or even as hemorrhage in the setting of diffuse metastasis [6–9]. Cholecystectomy, either open or laparoscopic, is routinely performed. The role of hepatic resection with or without lymphadenectomy in patients with this condition is an ongoing topic of discussion.
CASE REPORT
An 81-year-old male presented with the chief complaint of sharp right upper quadrant abdominal pain and nausea ongoing for 12 hours. The patient reported a similar, self-limited, episode 3 months previously. On presentation, leukocytosis 13 × 109/l, aspartate aminotransferase 83 U/l, alanine aminotransferase 49 U/l, lipase 78 U/l (normal 15–65), and total bilirubin 0.7 mg/dl. Abdomen and pelvis computed tomography (CT) demonstrated cholelithiasis in the gallbladder neck, soft tissue attenuation in the fundus of the gallbladder, mild extrahepatic biliary dilation and a common bile duct dilated to 10 mm (Fig. 1). Two well-circumscribed rounded filling defects within the gallbladder lumen, measuring 4.7 × 2.8 × 2.5 cm and 3.4 × 1.4 × 2.2 cm without distal shadowing, were visualized on right upper quadrant ultrasound (Fig. 2). CA 19-9 was negative (6.0 U/l). Magnetic resonance (MR) imaging was unable to be safely performed due to a metal prosthesis.
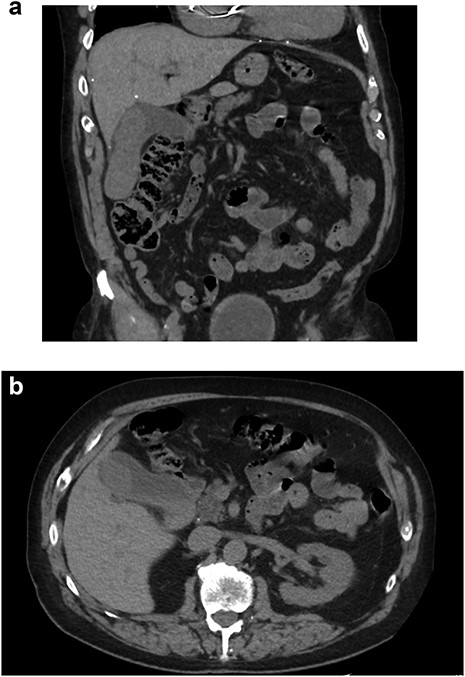
CT abdomen/pelvis without contrast. (A) Coronal and (B) axial slices depicting cholelithiasis, soft tissue within the gallbladder lumen and extrahepatic biliary ductal dilation
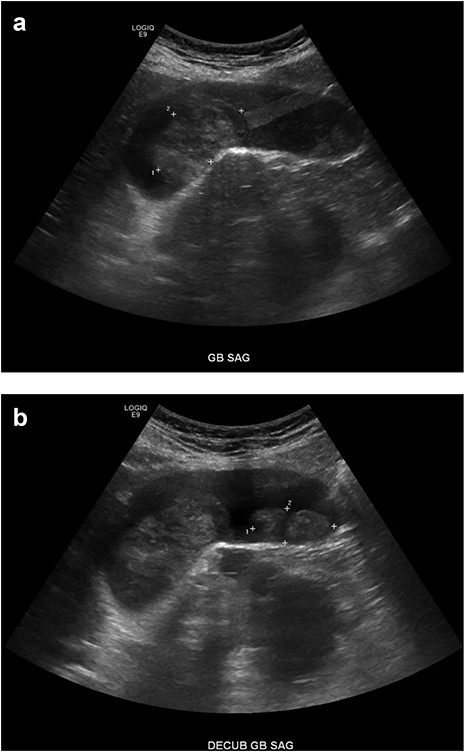
Right upper quadrant ultrasound. (A, B) A mildly distended gallbladder is visible containing two well-defined soft tissue masses within the gallbladder lumen, measuring 4.7 × 2.8 × 2.5 cm and 3.4 × 1.4 × 2.2 cm, respectively
The patient was taken to the operating theatre for laparoscopic cholecystectomy. The operative dissection was notable for evidence of acute and chronic cholecystitis with dense pericholecystic fibrous scarring, acute inflammatory changes and a severely distended gallbladder with decompression yielding purulent, hydropic fluid. Inadequate laparoscopic visualization of biliary anatomy necessitated conversion to open subtotal cholecystectomy. An accessory duct of Luschka, confirmed with intraoperative cholangiogram, was ligated. The patient recovered appropriately after surgery and was subsequently discharged from the hospital.
The operative specimen was sent for permanent pathologic evaluation. A polypoid intraluminal 5.5 cm mass microscopically demonstrated sheets and nests of malignant epithelioid neoplasm with focal elongated/spindle cell features, cytologic atypia, increased mitotic activity and necrosis, mixed with hemorrhagic tissue, without evidence of gallbladder wall involvement. The neoplastic cells stained positive for HMB-45, S100, SOX10, Melan-A, vimentin and BCL-2. SOX10 immunostaining on the cystic duct margin was negative. Molecular testing for t(12, 22) (EWSR1-ATF-1) translocation was negative. There existed no detectable V600 BRAF-type mutation.
Upon further discussion, the patient reported a history of cutaneous scalp melanoma excision several years prior to his admission. The patient was established with a medical oncologist and underwent positron emission tomography (PET) CT (Fig. 3), with focal uptake seen in the right hepatic lobe adjacent to the gallbladder fossa, concerning for residual disease versus postoperative changes. MR head was performed without evidence of intracranial metastasis. Repeat CT 5 months after resection was without evidence of progressive metastatic disease. This case was presented at an interdisciplinary tumor board conference; the consensus treatment plan was to pursue single-agent immunotherapy.
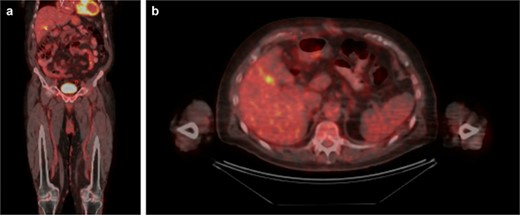
PET/CT. (A) Coronal and (B) axial slices depicting change status post cholecystectomy and focal uptake in the right hepatic lobe adjacent to the gallbladder fossa
DISCUSSION
Malignant melanoma diagnosed in the gallbladder presents a challenging treatment quandary. As stage IV disease, intraabdominal metastasis should be approached from a primarily palliative perspective given its poor prognosis. The most salient prognostic factor appears to be tumor biology rather than surgical treatment modality, as evidenced by retrospective analysis of patient survival versus metastatic dissemination. Surgical resection is uniformly considered a mainstay of treatment [7]. Open cholecystectomy is the optimal approach to resection, as trocar site recurrence has been described [10].
Adjuvant therapies are varied and of unclear overall potential. Interleukin-2 is well described in early immunotherapeutic treatments of metastatic melanoma, with the significant drawback of systemic toxicity. Patients without an identified driving mutation may benefit from the use of an anti-programmed cell death-1 antibody—a class including nivolumab and pembrolizumab. These can be used in concert with the anti-cytotoxic T-lymphocyte-associated antigen 4 antibody ipilimumab. BRAF inhibitors, vemurafenib and dabrafenib, are used with identification of the V600 mutation. Mutations in the KIT gene allow for the use of imatinib. The alkylating agent dacarbazine is the most common choice of cytotoxic chemotherapy [1].
Given the associated morbidity, treatment is ideally directed towards symptom control and preservation of quality of life. No standardized protocol exists in the treatment of this condition. As more cases are presented and treatment responses are described, an effort should be made to establish a validated treatment algorithm for this difficult problem.
Conflict of interest statement
None declared.



