-
PDF
- Split View
-
Views
-
Cite
Cite
Nicholas Mulchan, Alberto Cayton, Armand Asarian, Philip Xiao, Merkel cell carcinoma: a case report and literature review, Journal of Surgical Case Reports, Volume 2019, Issue 11, November 2019, rjz322, https://doi.org/10.1093/jscr/rjz322
Close - Share Icon Share
Abstract
Merkel cell carcinoma (MCC) is an aggressive cutaneous malignancy of neuroendocrine origin presenting as a painless, rapidly growing nodule. MCC often presents in elderly, fair-skinned individuals in sun-exposed areas. Diagnosis is often overlooked at time of presentation due to its rarity, but MCC is twice as deadly as malignant melanoma. There has been bigger interest in the disease due to increasing incidence and an association with the prevalent virus Merkel cell polyomavirus. This study describes an uncommon presentation of MCC as a right gluteal lesion in an Afro-Panamanian patient. The tumor was suspected to be fibrolipoma, but Immunohistochemistry revealed the diagnosis of MCC, as stains for CD56 and CK20 were positive. In addition to surgical excision, the patient was referred for adjuvant radiotherapy. This case report and literature review elucidates the clinical, histopathologic and management aspects of MCC, which will help in recognizing and treating these tumors.
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare but aggressive neuroendocrine cancer of the skin first described in 1972 as “trabecular carcinoma of the skin” [1]. The annual incidence of MCC worldwide is about 0.13–1.6 per 100 000 and rising [2]. MCC is believed to arise from Merkel cells in the basal layer of the epidermis and hair follicles and associated with nerve fibers [1]. However, other studies have suggested totipotential stem cells in the dermis or precursor B cells as the origin [1]. Risk factors for MCC include advancing age, light skin, immunosuppression, sun exposure and having another malignancy such as multiple myeloma or chronic lymphocytic leukemia (CLL) [1, 3]. Disease-specific mortality is 30%, which is twice that of malignant melanoma [1].
The majority of MCC cases are associated with monoclonal integration of Merkel cell polyomavirus (MCPyV), a non-enveloped, double-stranded DNA virus, into the host genome [4]. MCPyV is prevalent, with antibodies detected in 80% of individuals over 50 years old, but the only cancer it is associated with is MCC [1]. Remaining viral-negative cases of MCC are mostly associated with UV-related DNA mutations [1].
MCC usually presents in elderly, fair skinned individuals as a rapidly growing, painless, firm, red or flesh-colored nodule with a smooth surface [1, 4]. According to one study, tumors are often located in sun-exposed areas—head and neck (43%) and upper limbs and shoulder (24%). Patients present with local disease in 65% of cases, but many present with metastatic disease; 26% have regional lymph node involvement and 8% have distant metastases [5].
MCC is often confused with benign lesions such as a lipoma or epidermoid cyst and more common malignant lesions like basal cell carcinoma and amelanotic melanoma [4]. The acronym AEIOU has been developed based on a series of 195 cases over 27 years to describe MCC:
Asymptomatic
Expanding rapidly (weeks to months)
Immunosuppression (i.e. CLL, HIV, organ transplant)
Older than 50 years of age
UV-exposed area in a light-skinned person
The presence of three of these characteristics increases the likelihood of MCC [6]. However, the vagueness of these features stresses the importance of biopsy and histological analysis in confirming diagnosis.
CASE REPORT
An 80-year-old Afro-Panamanian male patient with no significant past medical history presented to the surgery clinic with a single painless, hard, hyperpigmented nodule on his right gluteal region that appeared 2 months ago and had been growing larger. The surgeon suspected simple fibrolipoma, so no initial imaging was deemed necessary.
After surgical excision, specimen was received by pathology in formalin and consisted of a fragment of pink-tan dense tissue measuring 6.5 × 5.5 × 4 cm. The cut section revealed a fresh and necrotic surface. Microscopic examination revealed nests of monotonous round tumor cells with infiltration of the subcutis. Tumor cells had a scant eosinophilic cytoplasmic rim and round, vesicular nuclei with abundant mitotic figures (Fig. 1) and neuroendocrine features (CD56 and Synaptophysin positive) (Fig. 2). Immunohistochemical stain CK20 demonstrated paranuclear dot-like staining, consistent with MCC (Fig. 3). Negative staining for CD45 excluded lymphoma. The tumor demonstrated positive margins with vascular invasion.
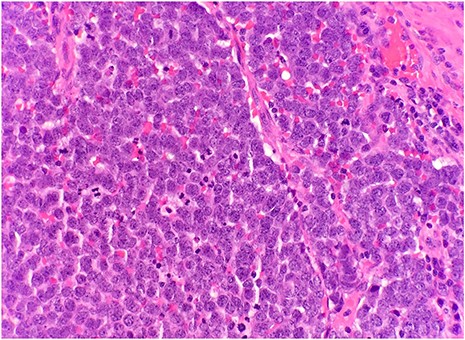
Microscopic examination reveals nests of monotonous round tumor cells. Tumor cells have scant eosinophilic cytoplasmic rim, round and vesicular nuclei with abundant mitotic figures (HE × 20).
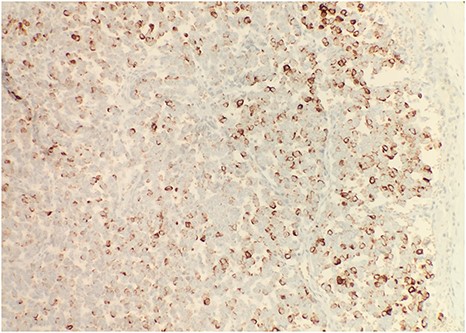
Immunohistochemical stain reveals that tumor cells are positive for synaptophysin (IHC × 20).
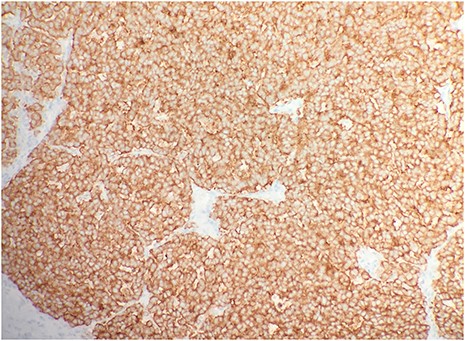
Immunohistochemical stain CK20 demonstrated paranuclear dot-like staining (IHC × 20).
The patient was brought back in a month after first surgery for wide re-excision, which resulted in clear margins. On follow-up exam, an enlarged right local inguinal node was found, which was excised two months after re-excision. Lymph node biopsy was positive. Positron emission tomography scan revealed no distant malignant lesion. Patient was referred to radiation-oncology for adjuvant radiotherapy (RT) to the tumor bed and nodal basin at a dose of 50 gray over 5 weeks; he is 1 week into RT to date.
DISCUSSION
MCC originates in the dermis, but can expand into the epidermis [1]. The tumor is composed of strands or nests of monotonous small round blue cells with prominent nuclei and little cytoplasm. The cells have frequent mitoses with “salt and pepper” chromatin [4]. Immunohistochemistry (IHC) is used for the definitive diagnosis of MCC. CK20 stains in a paranuclear dot-like pattern in 80–90% of MCC cases due to clumping of intermediate filaments. MCCs frequently stain positive for neuroendocrine markers, such as chromogranin, synaptophysin and CD56. MCC stains negative for TTF-1 and CD45, which distinguishes it from small cell lung carcinoma and lymphoma, respectively [1, 4].
While this case has the typical histopathologic characteristics of MCC, the clinical presentation is atypical. The incidence of MCC in the gluteal region, a non-sun exposed area, is less than 9% [5]. The few cases involving the gluteal region as a primary site that have been reported in English language literature are summarized in Table 1 below.[7–9] Furthermore, this is the first case of MCC reported in a Panamanian patient. The vast majority of patients (98%) affected by MCC are Caucasian [6]. The incidence and impact of MCC in the Latin American population has not been clearly described and requires further investigation [2].
| Year . | Age . | Sex . | Immunosuppression . | Management . |
|---|---|---|---|---|
| 20107 | 62 | M | Kidney–pancreas transplant | Surgery, ChT |
| 20118 | 60 | M | None | Surgery, adjuvant RT |
| 20167 | 76 | M | None | Surgery |
| 20187 | 73 | M | None | Palliative care |
| 20189 | 68 | M | None | Surgery, adjuvant RT, ChT |
| 20187 | 50 | M | Kidney transplant | Surgery, adjuvant/palliative RT, ChT |
| Present case | 80 | M | None | Surgery, adjuvant RT |
| Year . | Age . | Sex . | Immunosuppression . | Management . |
|---|---|---|---|---|
| 20107 | 62 | M | Kidney–pancreas transplant | Surgery, ChT |
| 20118 | 60 | M | None | Surgery, adjuvant RT |
| 20167 | 76 | M | None | Surgery |
| 20187 | 73 | M | None | Palliative care |
| 20189 | 68 | M | None | Surgery, adjuvant RT, ChT |
| 20187 | 50 | M | Kidney transplant | Surgery, adjuvant/palliative RT, ChT |
| Present case | 80 | M | None | Surgery, adjuvant RT |
| Year . | Age . | Sex . | Immunosuppression . | Management . |
|---|---|---|---|---|
| 20107 | 62 | M | Kidney–pancreas transplant | Surgery, ChT |
| 20118 | 60 | M | None | Surgery, adjuvant RT |
| 20167 | 76 | M | None | Surgery |
| 20187 | 73 | M | None | Palliative care |
| 20189 | 68 | M | None | Surgery, adjuvant RT, ChT |
| 20187 | 50 | M | Kidney transplant | Surgery, adjuvant/palliative RT, ChT |
| Present case | 80 | M | None | Surgery, adjuvant RT |
| Year . | Age . | Sex . | Immunosuppression . | Management . |
|---|---|---|---|---|
| 20107 | 62 | M | Kidney–pancreas transplant | Surgery, ChT |
| 20118 | 60 | M | None | Surgery, adjuvant RT |
| 20167 | 76 | M | None | Surgery |
| 20187 | 73 | M | None | Palliative care |
| 20189 | 68 | M | None | Surgery, adjuvant RT, ChT |
| 20187 | 50 | M | Kidney transplant | Surgery, adjuvant/palliative RT, ChT |
| Present case | 80 | M | None | Surgery, adjuvant RT |
Regarding management of MCC, surgery is the mainstay of treatment as well as adjuvant RT, which has been shown to reduce recurrence 3.7-fold [4]. Surveillance for recurrence with imaging and physical exams is recommended after initial therapy, especially in this case, as the lymphovascular invasion indicates a poor prognosis. This patient may become a candidate for further therapy if disseminated disease is discovered. While the role of chemotherapy in MCC has not been established, Avelumab, a monoclonal antibody that binds PD-1 was approved by the FDA in 2017 as first-line for those with metastatic disease [4, 10]. Clinical trials involving other immunotherapy targeting checkpoints PD-1, PD-L1 and CTLA-4 are taking place [1]. These immunotherapeutic strategies will have important implications for patients with advanced MCC.
In summary, this is a unique presentation of MCC in a non-immunosuppressed, Afro-Panamanian patient in a non-sun exposed primary site. It is important for clinicians worldwide to be cognizant of this uncommon presentation for prompt diagnosis and treatment given the aggressive nature of MCC.
Conflict of interest statement
None declared.



