-
PDF
- Split View
-
Views
-
Cite
Cite
José Maciel Caldas dos Reis, Fábio Akimaro Kudo, Moisés do Carmo Bastos, Fracture of a popliteal nitinol stent and pseudoaneurysm: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2019, Issue 11, November 2019, rjz312, https://doi.org/10.1093/jscr/rjz312
Close - Share Icon Share
Abstract
There is a lack of reports onr stent fracture with pseudoaneurysm formation in the femoropopliteal artery, which can cause restenosis or occlusion of the treated arterial segment. We present a case of a large pseudoaneurysm of the popliteal artery that was observed 18 months after popliteal stenting using a self-expandable nitinol stent. We describe an endovascular approach to overcome this severe complication. Stent fractures are an often overlooked complication of femoropopliteal stenting and can be associated with serious diseases. The popliteal artery was successfully treated using self-expandable Viabahn endoprosthesis.
INTRODUCTION
More than 50% of all obstructive lesions are located in the femoropopliteal segment, where they tend to be longer and have multiple coexisting atherosclerotic lesions at various levels [1]. Endovascular stenting of popliteal occlusions is a common, safe, and effective treatment, not only for obstructive diseases but also for nonobstructive conditions, such as prevention of formation of aneurysms [1, 2]. The introduction of nitinol stents with flexible geometry provides excellent patency results; however, stent fractures in the popliteal artery maybe asymptomatic and cause restenosis, false aneurysm, and acute thrombosis [2]. We present the case of a large false aneurysm of the popliteal artery, secondary to the fracture of a nitinol stent implanted 18 months previously. This case report aims to illustrate the complications of stent fractures in the popliteal artery, which was successfully treated with self-expandable Viabahn endoprosthesis.
CASE REPORT
Approval for retrospective reports is not required by our institutional review board. This study is in accordance with the principles of the Declaration of Helsinki. A 77-year-old man with multiple comorbidities, including coronary vascular disease, hypertension, dyslipidemia, previous right carotid endarterectomy, and endovascular aortic repair (in 2011), was referred to our unit for evaluation of a retropopliteal pulsatile mass. He had undergone infra-popliteal stenting 18 months earlier, to treat peripheral arterial disease with severe left claudication. The procedure was performed at another vascular institute, and the stent type was unknown. Physical examination revealed the presence of a femoral and popliteal pulse and the absence of tibial pulses; at admission, the ankle–brachial index was 0.8. According to the patient, he had noticed the mass in the last 2 months prior to admission, but the absence of pain delayed his presentation to the hospital. An ultrasound scan was performed on admission and revealed a large popliteal pseudoaneurysm with normal flow present distally, and fracture of the stent was confirmed by computed tomography, which also detected a 4.2 cm dilatation of the left popliteal artery (Figs 1–3).
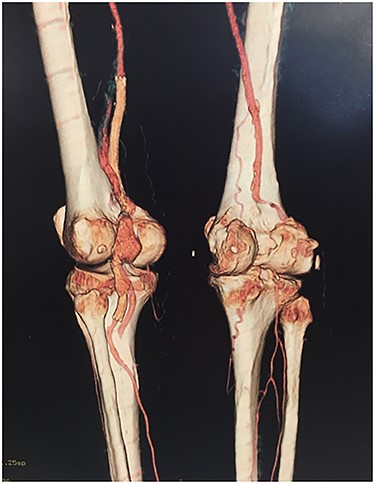
Angiotomography revealed fracture of a stent deployed in the popliteal artery and a pseudoaneurysm formation of size 4.2 cm at the part of stent fracture.
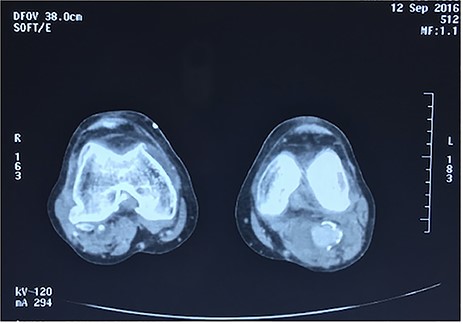
Angiotomography of the popliteal region highlighted the complete stent fracture and a large pseudoaneurysm formation.
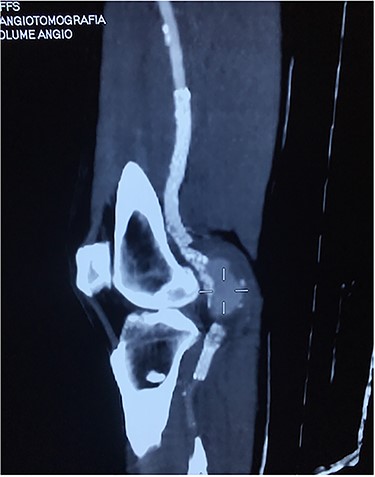
Angiotomography showing a fractured stent and pseudoaneurysm at the site of the fracture.
Based on the characteristics of the pseudoaneurysm, we decided on a minimally invasive interventional approach. This was in agreement with other vascular surgeons and the family. Under local anesthesia, the left common femoral artery was punctured in antegrade fashion, and an 8-F sheath was deployed. An X-ray of the popliteal region highlighted the stent fracture, and digital arteriography showed the large pseudoaneurysm. After administration of 5000 IU of heparin, a 0.035 guidewire was passed across the fractured stent and was placed distally in the posterior tibial artery. At the end of the procedure, a 7.0 × 150-mm self-expandable Viabahn endoprosthesis (W. L. Gore & Associates, Flagstaff, AZ, USA) was delivered into the stent, and adjuvant balloon inflation was performed with a 6.0 × 80-mm balloon leading to a complete exclusion of the pseudoaneurysm (Fig. 4). The final angiogram showed no residual stenosis, complete exclusion of the pseudoaneurysm, and no compromise of distal runoff. There were no complications associated with the procedure.
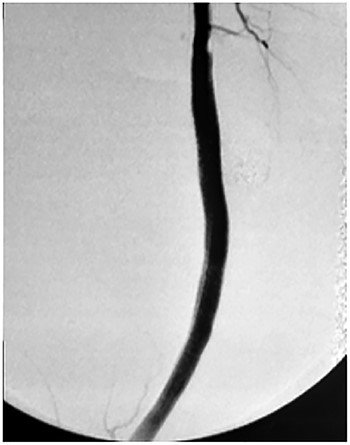
Viabahn endoprosthesis delivered after percutaneous intervention and immediately intraoperative angiogram.
DISCUSSION
More than 50% of all obstructive lesions are located in the femoropopliteal segment, and endovascular interventions are an alternative to open surgery in the treatment of peripheral arterial disease due to both superficial femoral and popliteal artery pathology [3]. Technical success rates of > 95% with low risk of complications have been achieved using angioplasty for anatomically suitable femoropopliteal lesions as they can be performed with minimal morbidity and mortality risk [4].
Nitinol, the material most commonly used in the manufacturing of stents, seems to confer with high biocompatibility and elasticity with shape memory properties. However, the super elasticity limits its rigidity and predisposes the stents to fracturing [2]. Over the years, significant changes have been undertaken in the structure and design of vascular stents, to improve their radial force, flexibility, and fracture resistance [5].
Stent fractures in the femoropopliteal segment are associated with long lesions, the number of stents used and may be asymptomatic, or they may lead to restenosis, thrombosis, pseudoaneurysm, or embolization resulting in both short- and long-term morbidity and mortality [5]. Excessive biomechanical deformations may explain the limited success of stents, in particular at the popliteal level [3].
Stent fractures in the femoropopliteal artery were clinically reported to be equally distributed throughout the femoropopliteal artery and first described by Duda et al. [6] who identified 6 of 33 (18.1%) cases in sirolimus coated cordis self-expandable stent (SIROCCO). Stent fractures may induce pseudoaneurysm formation; however there is a lack of reports. Previous studies have described interventional solution by endovascular stent grafting [7].
Placement of stents or endografts in the popliteal segment is generally not accepted due to anatomical and functional conditions specific to this segment, limiting long-term patency. Although the incidences of stent fractures differ according to stent types or the characteristics of lesion, fractures were noted to be most common in the femoropopliteal arteries, with a prevalence of 2–65% [8], which may lead to serious complications [3, 5]. During knee flexion, extensive kinking and functional stenosis may occur in the femoropopliteal segment. Furthermore, biomechanical stress and external mechanical forces from the surrounding musculature, including compression, torsion, and elongation, may lead to this deformation [9].
Normally, we use the self-expandable nitinol stent in the distal third of the superficial femoral and in the popliteal artery, because the structure of this stent is optimal for supporting the common forces induced by torsion and bending of this arterial segment [5]. Although there is a low rate of restenosis and fractures, requiring secondary procedures, a follow-up visit is crucial. At our institution the follow-up constitutes of ultrasound scans at 3 and 6 months after surgery and annually thereafter. The patient is required to undergo angiography, angiotomography, or angioresonance in case of symptoms or if the duplex ultrasound shows restenosis or other complications.
In conclusion, percutaneous techniques in the treatment of femoropopliteal obstructive lesions and the complication of femoropopliteal stenting causing pseudoaneurysm can be managed with self-expandable Viabahn endoprosthesis. Our case was successfully resolved with further endovascular intervention and the use of a stent graft with excellent patency at the 6-month follow-up.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest statement
No conflicts of interest are declared by the authors.
Funding
The authors have no sources of funding to report.



