-
PDF
- Split View
-
Views
-
Cite
Cite
Ulas Sozener, Transplantation of a horseshoe kidney from a living donor using stapler for transsection, Journal of Surgical Case Reports, Volume 2019, Issue 11, November 2019, rjz299, https://doi.org/10.1093/jscr/rjz299
Close - Share Icon Share
Abstract
Although it rather became a routine procedure to evaluate and use a cadaveric horseshoe kidney, using one from a living donor is quite rare. In this paper, we present methods we used during such a case which may benefit the procedures in the future. A 29-year-old female patient was considered for transplant and only viable living donor was her 59-year-old mother. Dynamic computed tomography revealed horseshoe anomaly with one renal artery and one renal vein for each side, a long but thin isthmus connecting lower poles with no visible arterial supply. Descending urography showed no connecting caliceal system. Donor nephrectomy was performed and isthmus separation was carried out with vascular stapler. Recipient was discharged on the 6th day with no complications. Patient was followed up for 6 months with normal creatinine levels. When properly assessed before the surgery, using a horseshoe kidney is not so challenging as thought.
INTRODUCTION
With the ever increasing number of end stage kidney failure patients, renal transplantation is still remains as the definitive treatment. Despite the advances in medical field, the number of donor organs is limiting the treatment. To compensate the increasing gap between demand and organ supply we have to expand our selection criteria for donor organs. Organs provided from deceased donors have no chance to meet the demand in countries like Turkey in the current state so we have to evaluate more and more living donors. Upon these evaluations, we started to encounter more marginal organs.
One of the most functional anomalies of the kidney is horseshoe abnormality. Although it rather became a routine procedure to evaluate and use a cadaveric horseshoe kidney, using one from a living donor is quite rare. Complex anatomical features, multiple vessels and complex collecting systems usually limit the surgeons to extract a portion without risking the functions of the remaining kidney.
In this paper, we present a case of horseshoe kidney form a living donor, our strategies and the methods we used during the operation, which may benefit the procedures in the future.
CASE REPORT
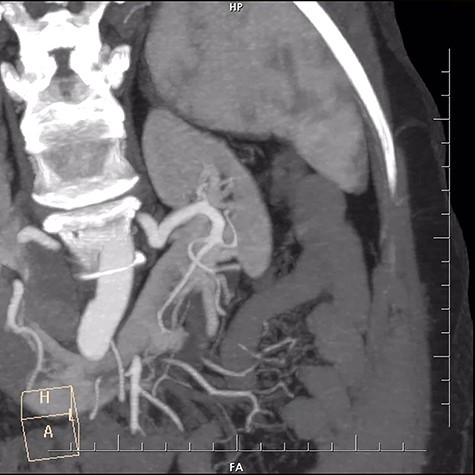
A 29-year-old female patient with end stage renal failure was our recipient. She was considered for a pre-emptive transplant and the only viable living donor was her 59-year-old mother. Upon evaluation for the donor, all the criteria were met except for the detected horseshoe kidney by CT scan. Dynamic CT revealed one renal artery and one renal vein for each side, a long but thin isthmus connecting lower poles with no visible arterial supply (Fig. 1). Sizes of the left and right kidneys were 110 × 38 cm and 117 × 42 cm, respectively. Absence of a connecting caliceal system was established with a descending urography. We did not perform a dynamic renal perfusion scintigraphy to assess the split functions of the kidneys since their sizes, respective vasculature and measured GFR values indicated sufficient capacity for each kidney. Left portion of the kidney was considered for nephrectomy.
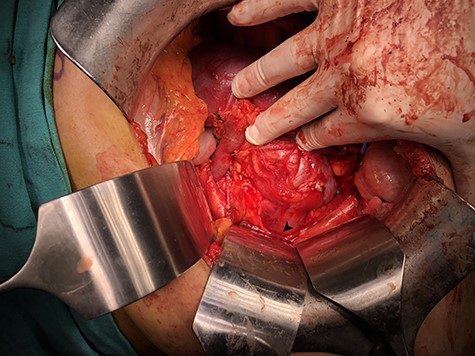
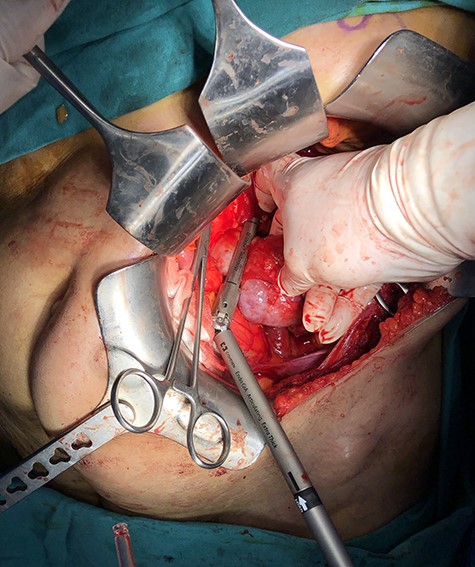
With a left subcostal incision transperitoneal exploration was performed. The left kidney had indeed one renal artery and one renal vein. There were two ureters for the left kidney. The vessels, ureters were prepared and the kidney was mobilized up to the isthmus. A temporary bulldog clamp was put on left renal artery to determine the demarcation zone and it was shown to form around isthmus (Fig. 2). The artery and vein were divided and the isthmus separation was performed with a 60-mm Endo GIA Black stapler (Medtronic, USA) (Fig. 3). After separation, no bleeding and no urine leakage were detected from transacted surface of the remaining kidney.
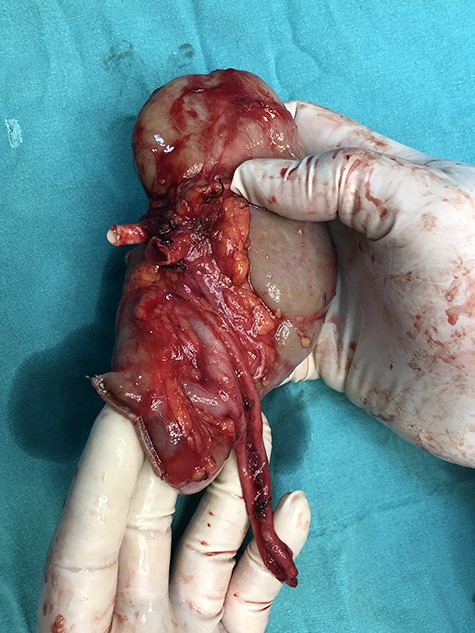
The graft was perfused with cold lactated ringer–prilocaine–heparin solution. There were no visible leaks (Fig. 4). It was transplanted to right iliac fossa of the recipient. Total cold ischemia duration was 21 min. Ureters were anastomosed to bladder separately side by side. Patient received tacrolimus, mycofenolic acid and methylprednisolone treatment. No collection was formed on the postoperative period, drain creatinine sampling showed no leakage and she was discharged on the 5th day with a serum creatinine level of 0.85 mg/dl. 6 months follow up showed a stable creatinine levels between 0.75 and 0.96 mg/dl.
No postoperative complications were observed in donor. Drain was taken out on the 3rd day after creatinine sampling and she was discharged on the 4th day. Her creatinine levels were stable during postoperative 1st, 3rd and 6th months follow-up 0.82, 0.86 and 0.85 mg/dl respectively. Her blood pressure levels, urine output, creatinine levels and GFR values showed a stable and sufficient renal capacity with remaining right kidney.
DISCUSSION
As previously discussed in similar studies, horseshoe kidney is one of the most common functional abnormalities of the kidney [1]. The size and function of the individual kidneys are almost always enough but the complexity of vascular and collecting systems easily frighten off the surgeons.
Most of the transplantation centres where the rate of transplantation is quite low, dealing with non-standard organs may prove a challenge, resulting in denying the donor.
With the proper preoperative imaging, the vascular structures are easily examined and the proper plane of dissection can be planned. We preferred clamping the artery, thus forming the demarcation zone to determine our border of dissection. Although use of methylene blue injection is another method, it was not necessary in our case [2].
We used a stapler to divide and seal the parenchyma of the kidney and it proved to be an easy and quick solution. As long as proper size of stapler with vascular sealing properties is selected, postoperative complications are rarely seen.
Horseshoe kidneys are used from deceased donors since 1975 [3]. Up to this date, this is the 10th case of living donor horseshoe kidney that has been successfully transplanted (Table 1). There were minor complications in some of them but overall the survival and the long-term function of the grafts were all favourable [4–10]. All the encountered complications are related to urinary system so we believe that most important part of preoperative planning is firmly establishing the caliceal relation of the kidneys. A descending urography will show the caliceal systems, it is easy to perform and cheap.
| Author . | Year . | Transplants . | Follow up (months) . | Complications . |
|---|---|---|---|---|
| Aikawa | 1998 | 1 | 20 | Urine leakage |
| Inoue | 2000 | 1 | 54 | Urine leakage |
| Goyal | 2003 | 2 | 18/12 | None/urine leakage |
| Hüser | 2005 | 1 | 16 | None |
| Dinckan | 2007 | 1 | 30 | None |
| Sezer | 2013 | 1 | 8 | Ureteral obstruction |
| Kumar | 2015 | 1 | Not reported | Not reported |
| Justo–Janeiro | 2015 | 1 | 24 | None |
| Sozener | 2018 | 1 | 6 | None |
| Author . | Year . | Transplants . | Follow up (months) . | Complications . |
|---|---|---|---|---|
| Aikawa | 1998 | 1 | 20 | Urine leakage |
| Inoue | 2000 | 1 | 54 | Urine leakage |
| Goyal | 2003 | 2 | 18/12 | None/urine leakage |
| Hüser | 2005 | 1 | 16 | None |
| Dinckan | 2007 | 1 | 30 | None |
| Sezer | 2013 | 1 | 8 | Ureteral obstruction |
| Kumar | 2015 | 1 | Not reported | Not reported |
| Justo–Janeiro | 2015 | 1 | 24 | None |
| Sozener | 2018 | 1 | 6 | None |
| Author . | Year . | Transplants . | Follow up (months) . | Complications . |
|---|---|---|---|---|
| Aikawa | 1998 | 1 | 20 | Urine leakage |
| Inoue | 2000 | 1 | 54 | Urine leakage |
| Goyal | 2003 | 2 | 18/12 | None/urine leakage |
| Hüser | 2005 | 1 | 16 | None |
| Dinckan | 2007 | 1 | 30 | None |
| Sezer | 2013 | 1 | 8 | Ureteral obstruction |
| Kumar | 2015 | 1 | Not reported | Not reported |
| Justo–Janeiro | 2015 | 1 | 24 | None |
| Sozener | 2018 | 1 | 6 | None |
| Author . | Year . | Transplants . | Follow up (months) . | Complications . |
|---|---|---|---|---|
| Aikawa | 1998 | 1 | 20 | Urine leakage |
| Inoue | 2000 | 1 | 54 | Urine leakage |
| Goyal | 2003 | 2 | 18/12 | None/urine leakage |
| Hüser | 2005 | 1 | 16 | None |
| Dinckan | 2007 | 1 | 30 | None |
| Sezer | 2013 | 1 | 8 | Ureteral obstruction |
| Kumar | 2015 | 1 | Not reported | Not reported |
| Justo–Janeiro | 2015 | 1 | 24 | None |
| Sozener | 2018 | 1 | 6 | None |
Another important point is to determine if the remaining kidney will be sufficient for the donor. Dynamic renal perfusion scintigraphies provide a good estimate for the functions of individual kidneys.
We can say that, when properly assessed before the surgery, using a horseshoe kidney is not so challenging as thought.
The important points are:
determining the function of both kidneys,
identifying the vascular variations,
proper and careful dissection of ureters due to not so rare occurrence of ureteric duplication and
splitting the kidney with proper care to avoid urinary leaks.
The waiting list for deceased donor kidneys continues to expand while the donation rate keeps forming a plateau in the last decade in our country. Living donations a good alternative but some patients have such a limited number of donors that we should customize our criteria to help those patients. Like in this case, denying the only available living donor of the patients means a long period of time in dialysis, which usually complicates the condition. Although use of horseshoe kidneys surely will not change the rate of supply and demand, it will be a game changer for some of those individual patients.
Conflict of interest statement
None declared.
Funding
None.



