-
PDF
- Split View
-
Views
-
Cite
Cite
Kazuomi Suzuki, Kiyoaki Taniguchi, Shunichi Ito, Akiko Serizawa, Masakazu Yamamoto, Leiomyosarcoma arising from the right ovarian vein, Journal of Surgical Case Reports, Volume 2019, Issue 11, November 2019, rjz302, https://doi.org/10.1093/jscr/rjz302
Close - Share Icon Share
Abstract
Leiomyosarcomas (LMS) of the ovarian vein are extremely rare and have a poor prognosis. Only 10 cases have been reported since 1977. The patient is a 69-year-old woman presented with right abdominal pain. Computed tomography showed a regularly shaped tumor, 80 mm in diameter in the retroperitoneum, adjacent to the descending part of the duodenum. Intraoperatively, the right ovarian vein was found to run through the tumor and was, therefore, resected together with the tumor. Pathological examination revealed a LMS of the right ovarian vein. Nine months postoperatively, multiple lung metastases were detected and chemotherapy was initiated. Delayed diagnosis is associated with high mortality. It is important that the diagnosis of LMS should be made preoperatively when you have already diagnosed a tumor to better direct the surgical approach. Multimodal therapy may improve prognosis.
INTRODUCTION
Tumors of vascular origin are rare, accounting for less than one in every 100 000 malignant tumors [1]. Leiomyosarcoma (LMS) is a rare tumor that affects smooth muscle tissue. Vascular LMSs most commonly originate from the inferior vena cava (IVC) [2]. Only a few cases of LMS arising from the ovarian vein have been reported in the literature. High metastatic potential is associated with the high mortality of vascular LMS.
CASE REPORT
A 69-year-old woman presented to our institution with right abdominal pain. There was nothing special to mention in her family history. Her past medical history included horseshoe kidney, gastric ulcer, and asthma. Physical examination revealed a slight tenderness of the right quadrant abdomen. All laboratory parameters including the tumor markers carcinoembryonic antigen and carbohydrate antigen 19-9 were within normal limits. Abdominal ultrasonography showed a regularly shaped uniform tumor of about 50 mm in diameter that was located in the right retroperitoneum ventral to the right part of the horseshoe kidney. The tumor was hypervascularized (Fig. 1). Computed tomography (CT) showed a tumor of 80 mm in diameter ventral to the right part of the horseshoe kidney and the dorsal side of the descending part of the duodenum. On contrast-enhanced CT, the tumor showed late-phase enhancement. There were no findings of invasion into any organs and right ovarian vein ran through the tumor (Fig. 2). No metastases to organs or swollen lymph nodes were found. Magnetic resonance imaging (MRI) showed a tumor that was isointense with respect to muscle on T1-weighted images and of high-signal intensity on T2-weighted images (Fig. 3). No fatty components were detected in the tumor. Endoscopic ultrasonography showed a regularly shaped and hypoechoic tumor with no connection to the right part of the horseshoe kidney or duodenum (Fig. 4). Our working diagnosis was a retroperitoneal tumor that could be either a malignant lymphoma, leiomyoma or gastrointestinal stromal tumor.
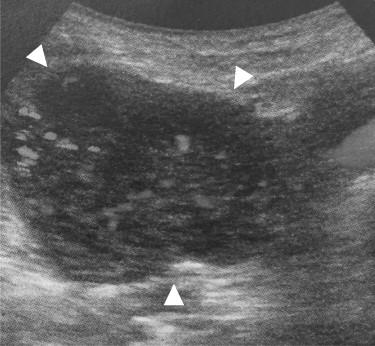
Abdominal ultrasonography: the tumor located lateral side of the right kidney, 40 mm in diameter (arrowhead). The tumor had much blood flow.
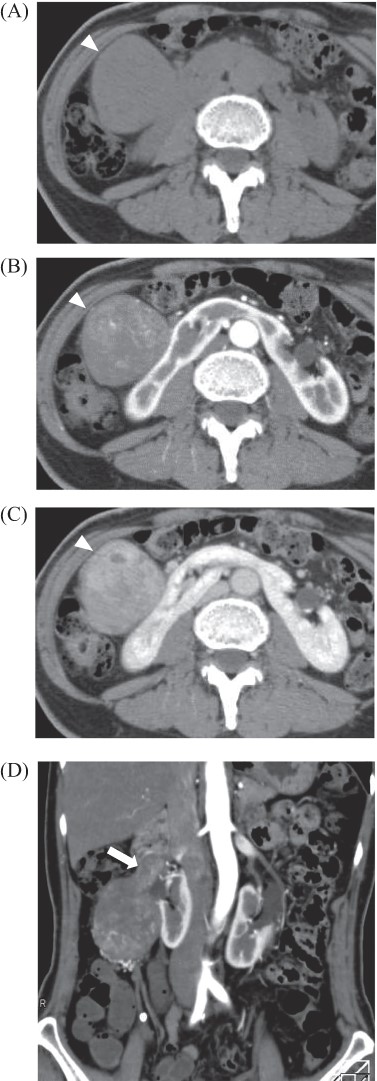
Abdominal enhanced computed tomography: there was a tumor, 80 × 40 mm in diameter at the ventral side of the right kidney and dorsal side of the duodenum, which was enhanced in late phase (A: plane; B: arterial phase; C: late phase, arrowhead). Tumor located in the right ovarian vein and tumor thrombosis was detected (D, arrow).
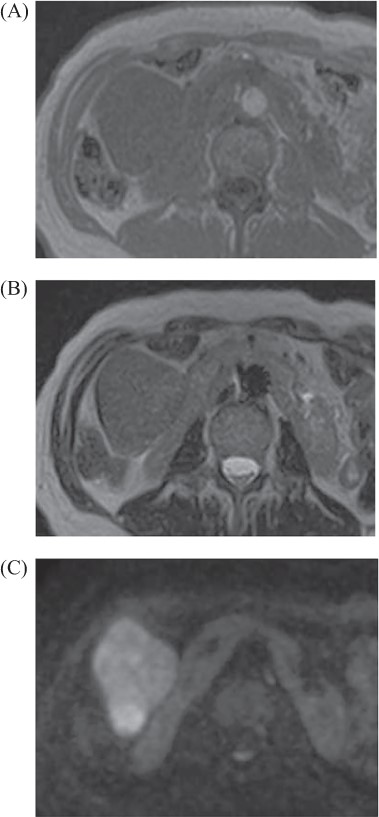
Abdominal MRI: tumor revealed iso intensity with muscle in T1 weighted image (A), slightly high intensity in T2 weighted image (B) and abnormal signal in diffuse weighted image (C).
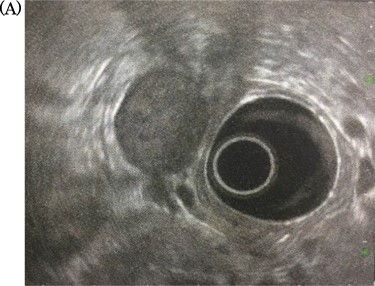
Endoscopic ultrasonography: there was no finding of infiltration to the right kidney and the duodenum.
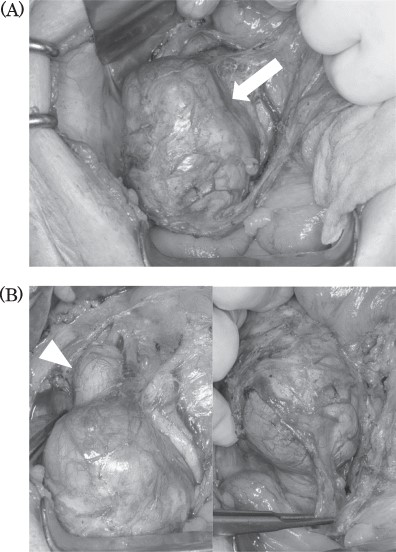
Intraoperative findings: tumor located in the retroperitoneum (A, arrow). Tumor did not invade to any other organs. Right ovarian vein throughout the tumor and tumor thrombosis was detected (B, arrowhead).
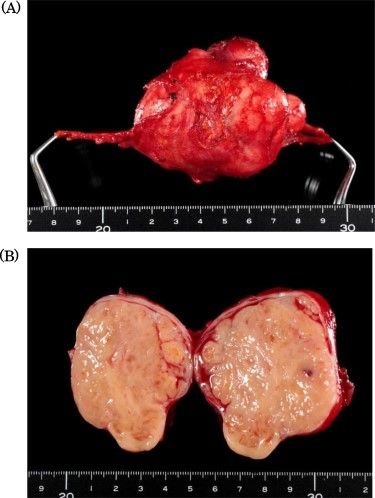
Specimen showed a solid tumor with white color, 80 × 40 mm in diameter.
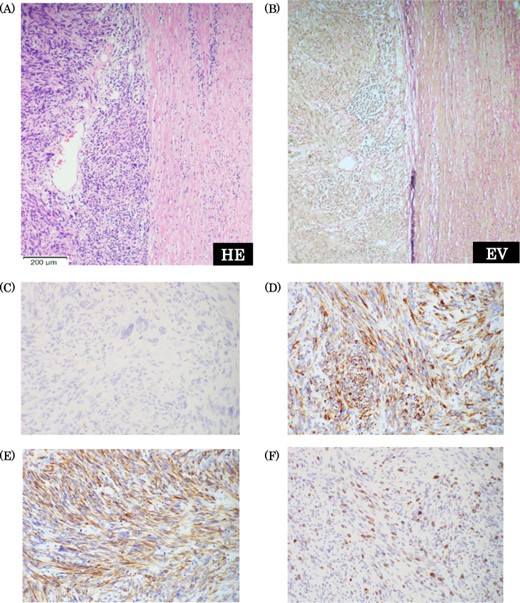
Pathological findings. (a) Hematoxylin-Eosin staining (×10): there were findings of high glade abnormal mitosis and proliferation of the spindle cells in fusiform. (b) Elastica van Gieson staining: there were component of elastic fiber in the tumor. (c) c-kit staining: negative. (d) Desmin staining: positive. e) Smooth muscle actin staining: positive. (f) Ki-67 index: 20%.
Intraoperatively, the tumor was located in the right retroperitoneal space and did neither adhere to nor invade other organs. The right ovarian vein ran cranially to caudally through the tumor. A central tumor thrombosis was detected in the ovarian vein (Fig. 5). We performed the resection of the tumor together with the right ovarian vein. The specimen showed a grayish-white solid tumor with the ovarian vein passing through its center (Fig. 6). Microscopically, fascicular hyperplasia of eosinophilic spindle cells with high-grade dysplasia and atypical mitotic figures were detected. Elastic fibers of the vessel wall were identified in the tumor. Immunostaining revealed that the tumor was positive for smooth muscle actin and desmin and negative for s-100 protein and c-kit. Based on these findings, our diagnosis was a LMS arising from the ovarian vein (Fig. 7). The immediate postoperative course was uneventful and the patient was discharged on day 11 postoperatively. Five months after the surgery, multiple lung metastases were detected on CT and chemotherapy was initiated (doxorubicin + olaratumab).
DISCUSSION
IVC is the main vascular LMSs in the retroperitoneal cavity. LMSs originate from smooth muscle cells in the tunica media of vessels and grow slowly, mostly extravascularly (60%). Intravascular and both extra- and intravascular patterns are few in numbers [3]. Most cases have initially no symptoms and are only detected when they cause abdominal pain or become palpable with increasing size.
LMSs arising from the ovarian vein were reported in only 10 cases since 1977 in the English literature. The median age in these patients was 55 years (range, 39–78). Six patients presented with abdominal pain, four with a palpable abdominal mass and one with genitourinary infection. The median size of the tumors was 8 cm (range, 4–21). Tumors tend to have already reached a considerable size when they are detected.
All of the 11 patients reported in the literature underwent surgical resection (Table 1): three had tumor resection only, eight extended resections (three included the ovarian vein, one the cecum, one a kidney and the ureter, one partial resection of the IVC and two hysterectomy and oophorectomy). Four patients (36%) suffered metastasis of their tumor. Common sites of metastasis are the lung and liver, usually through hematogenous spread. Vascular LMSs have a poor prognosis. The median overall survival (OS) is 5.5 years [1]. Italiano et al. [1] indicated that tumor size, tumor depth, grade and vascular origin were significantly associated with OS. The prognosis of vascular LMSs is worse than the prognosis of other organ LMSs because of the higher potential of hematogenous metastasis in vascular LMS. Further, detection at an advanced stage contributes to the poor prognosis. This applies particularly to LMSs of the ovarian veins that are located in the retroperitoneal space, where detection of tumors at an early stage is difficult. Some reports suggest that multidetector CT and 3D angiography are useful to identify the vascular origin of LMSs [4, 5].
Neither chemo- nor radiotherapy have been reported to be effective in LMSs, therefore surgical resection is the treatment of choice. Some reports indicated that perioperative radiotherapy does not improve the local recurrence rate of vascular LMSs [6]. Hensley et al. [7] conducted a prospective study comparing adjuvant chemotherapy (gemcitabine plus docetaxel followed by doxorubicin) with observation only after resection of uterus LMSs and did not find an effect of chemotherapy. Aiba et al. [8] suggested that radio-hyperthermo-chemotherapy is an effective option for salvage treatment of recurrent and residual soft tissue sarcoma. In case of tumor infiltration into other organs, extended resection is recommended. Roberts et al. [9] refer to the potential effectiveness of other treatments such as targeted therapy (platelet-derived growth factor inhibitors; olaratumab and tyrosine kinase inhibitors; pazopanib) and immunotherapy (anti-PD-1 antibodies; nivolumab, pembrolizumab).
Because there are only a few cases of LMSs of ovarian vessels described in the literature, case reports like this one are important to gain a better understanding of the characteristic features of these rare tumors.
Conflict of interest statement
None declared.



