-
PDF
- Split View
-
Views
-
Cite
Cite
Yahya Alwatari, Wayne Tse, Kasia Trebska-McGowan, Rachit D Shah, Use of endoloop in video-assisted thoracoscopic enucleation of a very rare esophageal tumor, Journal of Surgical Case Reports, Volume 2019, Issue 11, November 2019, rjz300, https://doi.org/10.1093/jscr/rjz300
Close - Share Icon Share
Abstract
A gastrointestinal stromal tumor is an infrequent tumor of the gastrointestinal tract with very rare involvement of the esophagus. We present a case of a patient with dysphagia and a 4 cm submucosal mass. The patient underwent thoracoscopic enucleation with complete resection of the mass. We present case details and operative video highlighting the important surgical steps of exposure and retraction. We believe that the Endoloop is a very useful tool in providing countertraction needed during minimally invasive resection of such lesions.
INTRODUCTION
A gastrointestinal stromal tumor (GIST) is the most common sarcoma that arises in the GI tract, although its occurrence is rare with a reported incidence at approximately seven cases per million [1]. Studies have also shown GIST to be a result of a gain-of-function mutation in c-KIT with positive immunohistochemical staining for CD34 and, the more specific marker, CD117 [2]. This immunophenotype suggests that GISTs are derived from interstitial cells of Cajal, and originate in the stomach (50–60%), small intestine (25–30%), colon/rectum (1–2%), with ~1% originating in the esophagus [3]. Due to its rarity, optimal management of esophageal GIST remains to be delineated.
CASE REPORT
The patient is a 67-year-old male with a history of atrial fibrillation and hyperlipidemia who presented with several months of dysphagia to solids. The endoscopic evaluation revealed a ~4 × 2.3 cm submucosal lesion arising from the muscularis propria in the distal esophagus (Fig. 1). The lesion was 2 cm proximal to the GE junction and intermittently disappearing with peristaltic contractions. The lesion was biopsied and showed low-grade GIST with 1–3 mitoses per high power field and was CD 117 positive. Computerized tomography (CT) scan showed a right esophageal mass without evidence of local invasion (Fig. 2).
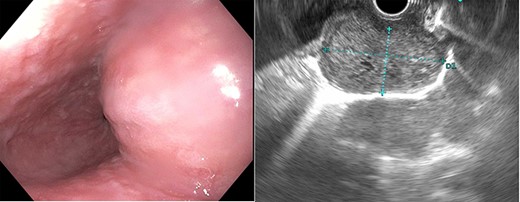
Esophagogastroduodenoscopy showed an esophageal prominence 2 cm above the GE junction with endoscopic ultrasound confirming 39.8 × 23.2 mm mass.
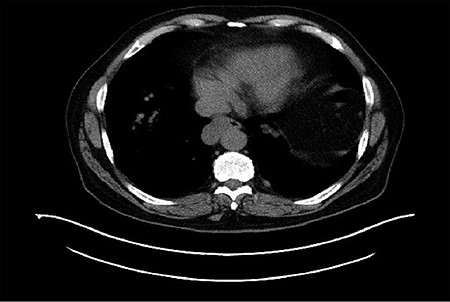
Computerized tomography (CT) demonstrated a right distal esophageal mass.
After discussing risks and benefits with the patient and obtaining informed consent, the decision was made to proceed with surgical resection. An upper endoscopy was performed confirming the findings seen before. The patient was intubated with a dual lumen endotracheal tube and placed in a left lateral decubitus position. Standard video-assisted thoracoscopic ports were placed. The inferior pulmonary ligament was divided allowing the lung to be reflected away. Approximately ~4 cm lesion was visualized in the distal esophagus. Using an endoscopic harmonic scalpel, the pleura was incised and adhesions to the right lower lobe were dissected.
The lesion was carefully dissected away from the surrounding muscular layers of the esophagus. An anchoring suture was first placed at the superior pole of the mass as an attempt to provide countertraction, which failed due to the fragility of the lesion. Countertraction was better achieved with an Endoloop Ligature (Ethicon Inc, New Brunswick, NJ) placed around the basal portion of the lesion. Care was taken to gently pull on the Endoloop without overtly tightening it and inadvertently cutting through the lesion. The mass was then completely enucleated and separated from the esophageal mucosal layer. The highly vascular tumor was prone to intermittent bleeding and was controlled with a combination of pressure and Bovie electrocautery. The specimen was removed through a laparoscopic specimen bag. The esophageal muscle layer was reapproximated with interrupted sutures. Repeat upper endoscopy confirmed the integrity of the esophageal mucosal layer. The patient underwent an esophagogram on post-operative day one prior to oral intake and discharged home later that day. Final pathology confirmed a 4.7 cm esophageal GIST with <5 mitoses per high power field and positive for CD 117 and 34.
DISCUSSION
A total of 76% of patients with esophageal GIST are symptomatic at the time of diagnosis, most commonly presenting with dysphagia, weight loss, hemoptysis, abdominal pain, nausea, cough, vomiting, gastroesophageal reflux and night sweats [4]. Visualization of GIST can be achieved through endoscopy, but definitive diagnosis before considering a resection can sometimes be difficult. While 18F-fluorodeoxyglucose positron emission tomography has been used to describe the malignancy of GIST, results cannot discriminate the tumor from other benign masses in the esophagus [5]. Similarly, esophageal ultrasound and CT scans cannot make this distinction either. The only effective method of distinguishing esophageal GIST from other mesenchymal tumors is ultrasound-guided fine-needle aspiration biopsy, which allows for histologic examination of the tumor and immunohistochemical staining.
The current standard of care for treatment of GIST is surgical resection with the aim to achieve negative tumor margins. Studies have shown that adjuvant therapy with tyrosine kinase inhibitors can improve recurrence-free survival in localized GIST. Unfortunately, these studies did not include esophageal GIST, thus Imatinib therapy needs to be further explored with this subtype [6]. Although currently experimental, it is important to consider neoadjuvant therapy with pre-operative Imatinib when treating a large esophageal GIST, as the reduction in tumor size caused by tyrosine kinase inhibitors may decrease the risk of tumor rupture and optimize resection [7]. Patients with high-risk features should be considered for adjuvant Imatinib therapy.
Surgical options for esophageal GIST include minimally invasive vs open esophagectomy or enucleation based on tumor size and location. Enucleation is preferred for patients with comorbidities and with small tumor size while esophagectomy is indicated for larger tumor sizes. Zheng et al. [8] reported the effectiveness of using Endoloop assistance in endoscopic enucleation of deep GIST gastric tumors. We believe that it should be considered as a useful adjunct for countertraction in video-assisted enucleation of esophageal GIST lesions. Gentle and stable traction provided by the Endoloop can aid in better visualization of the tissue planes between the esophageal mucosa, lesion, and the muscle layer and help in quicker and safer completion of the case. The use of Endoloop for retraction has been reported in other minimally invasive surgeries [9]. It has the advantage of the ease of use and low risk of instrumental perforation during retraction. The other advantage of Endoloop is their relatively low-cost compared to other retraction devices and small diameter making their use through the same port with other instruments possible; however, instrumental positioning and re-positioning can be difficult with Endoloop [9] and it requires obtaining a circumferential plane around the retracted structure. Recently, Funamizu et al. [10] reported a series demonstrating the efficacity and safety of Endoloop use in gallbladder retraction in single-incision laparoscopic cholecystectomy.
In conclusion, esophageal GISTs are very uncommon and are amenable to thoracoscopic enucleation. This allows for lower morbidity with an overnight hospital stay and a quick return to normal activities. The use of Endoloop for countertraction provides excellent exposure of the underlying esophageal muscle and mucosal layers.
AUTHORS’ CONTRIBUTIONS
Y.A., W.T., K.T.-M. and R.D.S. were responsible for data collection, study design and writing the manuscript. R.D.S. supervised the project.
DISCLOSURE/CONFLICT OF INTEREST
None declared.



