-
PDF
- Split View
-
Views
-
Cite
Cite
Lorenzo Cocchi, Stefano Di Domenico, Sergio Bertoglio, Elio Treppiedi, Gianluca Ficarra, Franco De Cian, Inferior vena cava resection without reconstruction for retroperitoneal malignancies, Journal of Surgical Case Reports, Volume 2019, Issue 10, October 2019, rjz275, https://doi.org/10.1093/jscr/rjz275
Close - Share Icon Share
ABSTRACT
Inferior vena cava (IVC) involvement in retroperitoneal malignancies is a rare occurrence and radical surgery with major vascular resection represents the only potential curative treatment. IVC replacement after resection is still controversial and only small series and few prospective data are available. We report a series of three patients affected by retroperitoneal masses involving IVC treated with vena cava resection without replacement. All patients were treated by a radical R0 surgical procedure associated with infrarenal IVC resection and no reconstruction. Based on preoperative radiologic imaging and intraoperative findings, one patient also underwent right nephrectomy, while another patient underwent left renal vein ligation without nephrectomy. Neither early nor late severe post-operative complications related to the absence of IVC outflow were observed. Resection without replacement of the infrarenal IVC results in acceptable morbidity, thus specific risks related to the use of prosthetic grafts can be avoided.
INTRODUCTION
Retroperitoneal tumors are an heterogeneous group of diseases, and malignant lesions are more frequent than benign lesions. Renal cell carcinomas, retroperitoneal liposarcomas, leiomyosarcomas and retroperitoneal metastatic lymph nodes from testicular carcinoma represent the most common malignancies of this district. Radical surgery (R0) represents the only potential curative treatment, even if the prognosis remains poor. Inferior vena cava (IVC) involvement is uncommon and, when occurs, major vascular resection may be necessary to achieve radical resection with negative margins. IVC reconstruction after resection is still controversial.
CASE REPORTS
The authors report three consecutive cases of patients affected by retroperitoneal malignancy with IVC involvement who were treated at our Institution with radical R0 resection.
Case 1 was a 33-year-old male affected by a right testicular germ-cell tumor with enlarged retroperitoneal lymph nodes surrounding the infrarenal IVC. The patient underwent orchifunicolectomy and subsequent chemotherapy based on bleomycin, etoposide and platinum. A restaging computed tomography (CT) scan showed partial response and confirmed residual tumor involving both the IVC (with occluding thrombus) and the distal left renal vein (LRV) (Fig. 1A). CT scan also showed an efficient venous collateral pathway through the azygos−lumbar system (Fig. 1B), with no clinically evident lower limb edema. The patient underwent an en-bloc resection of the tumor mass with infrarenal IVC and LRV ligation without nephrectomy; no IVC prosthetic replacement was carried out. Recovery from surgery was uneventful with the exception of transient mild renal failure treated conservatively and without the need for dialysis. Renal ultrasonography showed regular bilateral parenchymal perfusion, and doppler ultrasonography of the lower limbs confirmed normal superficial and deep venous outflow. The patient was discharged 9 days after surgery. Histology showed a teratoma with alpha-fetoprotein expression and extensive post-chemotherapy areas of necrosis.
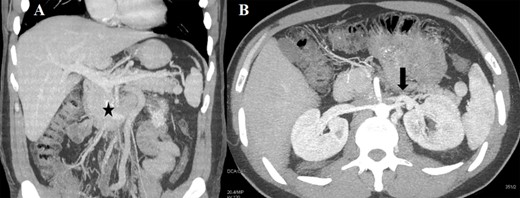
(A) CT scan (coronal section) of the retroperitoneal mass (asterisk) involving the IVC and LRV with congested gonadal vessels; (B) cross-section CT scan showing well-represented collateral outflow (arrow) of the renal–azygos–lumbar system.
Case 2 was a 23-year-old male with a testicular germ-cell tumor and evidence of pathologically enlarged abdominal and cervical lymph nodes. After surgical excision of the primary tumor and administration of four cycles of Bleomycin, Etoposide and Platinum (cisplatin) (BEP) + four cycles of paclitaxel, ifosfamide and cis-platinum at another institution, the patient was referred to us. CT scan showed stable disease with a large infrarenal inter-aortocaval mass (13 × 10 cm) obstructing the IVC (Fig. 2A). Extensive abdominal lymphadenectomy and resection of the infrarenal IVC without prosthetic replacement was performed (Fig. 2B). Concurrently, ear−nose and throat surgeons performed modified radical left neck lymphadenectomy. The post-operative course was characterized by transient renal failure and moderate anemia requiring blood transfusions and rehydration therapy. The patient was discharged 13 days later and had normal renal function values and no relevant inferior limb edema. Histology confirmed the diagnosis of lymph node metastases of the germ cell tumor.
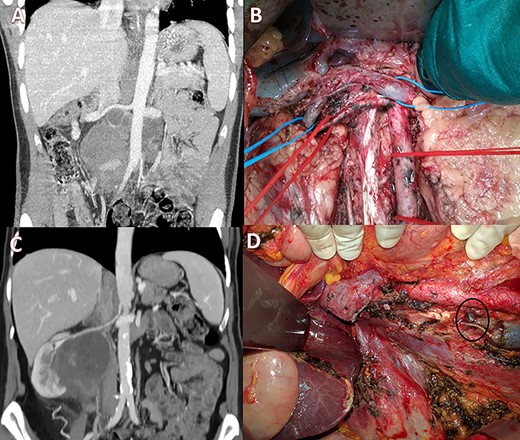
(A) Pre-operative CT scan with a large lesion involving the IVC; (B) intraoperative view showing ligation of the IVC with renal-vein preservation; (C) coronal CT scan with a huge retroperitoneal space-occupying lesion infiltrating the right kidney and occluding the IVC; (D) intraoperative image after right nephrectomy and IVC resection showing a large collateral lumbar trunk (circle).
Case 3 was a 65-year-old woman with a 9 × 7 cm retroperitoneal mass infiltrating the right kidney and the IVC with radiologic evidence of IVC and right ureteral obstruction (Fig. 2C). Percutaneous biopsy was compatible with angiomyosarcoma. Clinical examination showed no edema of the lower limbs. Neo-adjuvant chemoradiotherapy (doxorubicin + dacarbazine followed by 50 Gy in 25 fractions) was performed. Post-treatment positron emission tomography scan imaging showed stable disease with partial metabolic response. Subsequently, the patient underwent radical resection of the tumor with right nephrectomy and infrarenal IVC resection. No IVC reconstruction was performed and at surgery, venous outflow through a collateral venous lumbar vein was noticed and preserved (Fig. 2D, circle). No major perioperative complications occurred. Post-operative ultrasonography revealed normal lower limb venous pathways and the patient was discharged on post-operative day 11. Histological examination confirmed a leiomyosarcoma grade 2 Fédération Nationale des Centres de Lutte Contre Le Cancer (FNCLCC criteria) with large post-chemotherapy areas of necrosis.
DISCUSSION
Involvement of the IVC in retroperitoneal malignancies is an uncommon event. The most often represented tumors in these clinical situations are renal cell carcinomas followed by leiomyosarcoma and testicular cancer [1]. Prognosis is generally poor and the only possible curative treatment is R0 resection with associated vascular resection if needed [2–4]. Due to the rarity of this clinical presentation, there is no unanimous agreement about the surgical management of the IVC resection. Tumors involving less than 180° of the vascular wall are usually managed by venous tangential resections. Circumferential resections may be necessary in some cases in order to achieve oncological radicality and in particular, vascular replacement with homo- or allograft is usually performed in case of suprarenal IVC resection [5]. On the contrary, surgery for infrarenal IVC involvement is more frequently managed by venous resection without venous reconstruction [5, 6]. This approach may be taken into consideration in order to reduce the likelihood of life-threatening, graft-related complications such as early or late thrombosis, infections or anastomotic leakage. The presence of a complete preoperative IVC occlusion, the absence of lower limb swelling at clinical presentation, or radiologic or intraoperative evidence of venous collateral outflow through a renal–azygos–lumbar pathway should be evaluated before vena cava prosthetic replacement [7, 8]. The advantage of avoiding vascular graft replacement may be considerable, especially in patients undergoing multiorgan resection or in those being treated with neoadjuvant chemo- or chemo-radiation therapy, which are the risk factors for post-operative complications. Although significant prospective data are not available due to the rarity of this scenario, various case series are in favor of this technique [5, 6, 8]. In accordance with these reports, our three cases have shown an acceptable degree of morbidity without long-term sequela or mortality and with a median hospital stay of 11 days. The six month follow-up visits showed no signs of deep vein thrombosis or renal failure in any of the three patients. In conclusion, although our experience was related to a limited number of patients, we believe that in carefully selected patients, infrarenal IVC ligation after resection is a safe procedure and may reduce graft-related complications.
Conflict of interest
The authors have no conflict of interest or source of funding.
References
- postoperative complications
- intraoperative care
- ligation
- nephrectomy
- preoperative care
- reconstructive surgical procedures
- retroperitoneal space
- surgical procedures, operative
- inferior vena cava
- vena cava
- diagnostic imaging
- morbidity
- radiology specialty
- left renal vein
- prosthetic grafts
- malignant retroperitoneal tumor



