-
PDF
- Split View
-
Views
-
Cite
Cite
Bojana Misheva, Roy Hajjar, Frank Schwenter, Jocelyne Martin, Herawaty Sebajang, Gastric necrosis late after a Collis-Nissen procedure, Journal of Surgical Case Reports, Volume 2019, Issue 10, October 2019, rjz272, https://doi.org/10.1093/jscr/rjz272
Close - Share Icon Share
Abstract
A Nissen procedure is an efficient surgical approach to treat gastroesophageal reflux disease. It is sometimes combined with a Collis gastroplasty to lengthen the functional distal esophagus to allow a 360° Nissen fundoplication without tension. We present the case of a 76-year-old female patient, with a history of a Collis-Nissen procedure, who developed extensive gastric necrosis after ingesting a significant quantity of maize. She underwent an urgent total gastrectomy with Roux-en-Y esophagojejunostomy. The cause of ischemia and necrosis in this case is believed to be an insufficient blood supply due to excessive intraluminal pressure. Necrosis of the gastric cavity is usually more likely to be due to venous insufficiency as veins’ walls are less resistant to compression than arterial vessels. Gastric necrosis after a Collis-Nissen procedure is exceedingly rare, and symptoms of such a complication are usually vague and not pathognomonic, which might delay surgical care and increase morbidity.
INTRODUCTION
The Nissen fundoplication was introduced in the mid-twentieth century to treat gastroesophageal reflux disease and hiatal hernias. A Collis gastroplasty aims to lengthen the functional distal esophagus to allow a 360° Nissen fundoplication without tension. This procedure has greater than 90% success in eliminating reflux symptoms, but some patients develop over time recurrent dyspepsia [1]. Rarely, a Nissen fundoplication can potentially create an upper gastric outlet obstruction, which may potentially induce ischemia when the stomach is severely distended and unable to empty properly [1].
CASE PRESENTATION
We present the case of a 76-year-old female patient who developed extensive gastric necrosis after a Collis-Nissen procedure. Her past medical history includes hypertension, dyslipidemia, chronic pulmonary obstructive disease and obesity (body mass index of 33 kg/m2). The patient had suffered 1 year prior to the present episode from a gastric volvulus due to an incarcerated hiatal hernia. At that time, she underwent a thoracotomy and a Collis-Nissen procedure, from which she recovered uneventfully.
She presented a year later at a peripheral hospital with severe abdominal pain awakening her during the night. The episode was preceded by a few days of diffuse abdominal discomfort. An abdominal computed tomography (CT) scan was performed and showed a pneumoperitoneum and a distended stomach. The suspected diagnosis was a perforated gastric ulcer. An exploratory laparotomy was performed. The gastric wall was noted to be diffusely necrotic with two perforation zones in the lesser curvature. Due to the previous gastric surgery and the significant gastric injury that was noted to be present, a decision was made to transfer the patient into our referral hospital with intraperitoneal Jackson-Pratt drains and a vacuum-assisted closure device containing the viscera.
The patient arrived intubated in a stable condition. She was put on wide-spectrum antibiotherapy of piperacillin/tazobactam. Thoracic and abdominopelvic CT scans were performed at our institution and showed pneumatosis in the posterior wall of the gastric cavity as well as in the antrum wall and the lesser curvature, suggestive of gastric ischemia (Fig. 1). There was no evidence of arterial or venous thrombosis of major abdominal and thoracic vessels. The gastric injury was extensive, and the stomach deemed unsalvageable. The patient underwent a total gastrectomy with Roux-en-Y esophagojejunostomy. A feeding jejunostomy was installed intraoperatively.
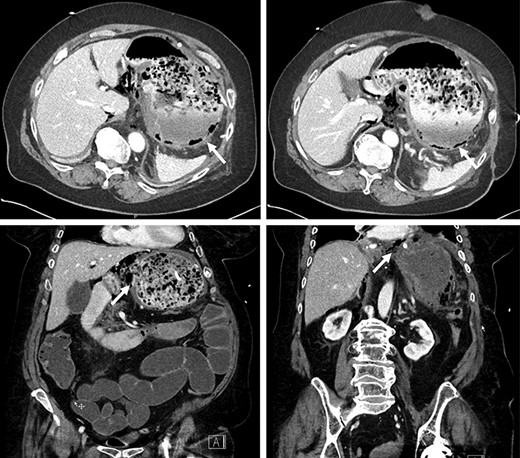
Pneumatosis in the posterior wall and lesser curvature of the gastric cavity on CT scan.
Histopathological examination of the specimen showed a transmural ischemic necrosis of the gastric wall. There was no evidence of a neoplastic process.
The patient mentioned after surgery that her symptoms started after ingesting a significant quantity of maize. In the postoperative period, the patient developed a fungemia to Candida glabrata that was treated accordingly. No intra-abdominal complications occurred. A barium swallow was performed in the postoperative period to assess the integrity of the esophagojejunal and jejunojejunal anastomoses. No leak was objectified (Fig. 2). The patient was successfully discharged home after a hospital stay of 20 days. The feeding jejunostomy tube was removed 2 months later. No outpatient complications were noted except mild deconditioning that required physical therapy.
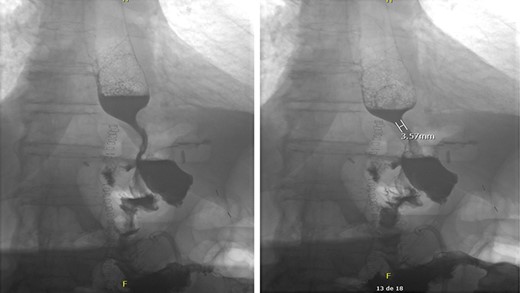
Intact esophagojejunal and jejunojejunal anastomoses on barium swallow.
Due to inadequate oral intake and weight loss, an upper gastrointestinal endoscopy was performed 1 year later. An anastomotic stricture was objectified, for which a stent was installed (Fig. 3). The stent was removed 6 weeks later, and the width of the anastomosis was appropriate (Fig. 4).
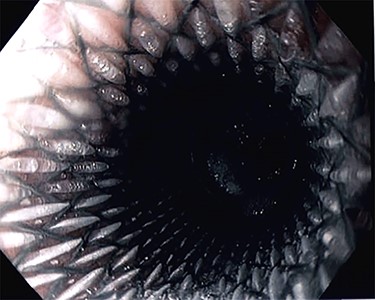
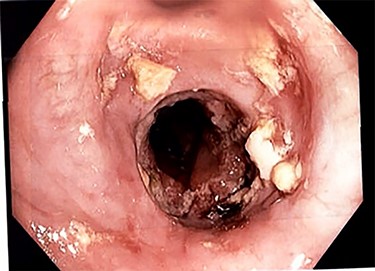
Esophagojejunal anastomosis after removal of the endoscopic stent.
DISCUSSION
Acute gastric dilation (AGD) may be a result of psychogenic polyphagia, electrolyte abnormalities or hiatal hernias complicated by volvulus or intestinal obstruction [2]. Early symptoms are usually deceptively vague [2]. Patients may present with mild abdominal discomfort, vomiting or abdominal distention. Signs of peritoneal irritation and hemodynamical instability are present when a complication occurs and are thus a sign of an advanced illness. A fearsome complication of AGD is ischemia and necrosis due to an insufficient blood supply that is impaired by excessive intraluminal pressure. The stomach is supplied by a rich collateral circulation making the organ resistant to ischemia. Necrosis of the gastric cavity is thus more likely to be due to venous insufficiency as veins’ walls are less resistant to compression than arterial vessels. For the arterial blood supply to be compromised, the intraluminal pressure of the organ needs to be greater than 20–30 cm H2O [3].
To the best of our knowledge, very few cases of gastric necrosis after a Nissen fundoplication have been reported, with the cause being usually an acute obstruction of the small bowel due to adhesions, ileus or a gastric outlet obstruction due to malignancies or bezoars [4–7]. It was hypothesized that the “gas-bloat syndrome,” a known postoperative complication of the Nissen fundoplication, may lead to AGD. In the “gas-bloat” syndrome, patients usually complain of abdominal bloating, postprandial fullness, nausea, flatulence, inability to vomit, and epigastralgia [8]. One of the explanations for this syndrome is the one-way valve effect that is created by the fundoplication to prevent gastric reflux. When the anterograde transit is altered, expelling gastric content or trapped air becomes impaired, resulting in a progressive gastric dilation [8].
The exact cause of the gastric necrosis in our patient is most probably due to a closed-loop obstruction of the gastric cavity. The upper obstruction was caused by the Nissen procedure, and the distal occlusion was most probably due to a bezoar formation caused by the important quantity of high-fiber food she ingested in the days prior to her admission. No vascular thrombus was identified, nor a malignant process that may have caused a gastric outlet obstruction. The necrosis extended beyond the gastric wrap of the fundoplication so a local necrosis by arterial insufficiency is not likely.
A high index of suspicion for AGD in patients who have undergone previous anti-reflux surgery is critical to avoid further complications. Management with nasogastric (NG) decompression is suggested as the initial management. The placement of a NG decompression tube could be however challenging due to the narrow fundoplication site. If there is concern for potential ischemia on imaging, surgical exploration is warranted as gastric necrosis is associated with poor outcomes. When gastric necrosis is encountered, gastrectomy is typically indicated with immediate or delayed reconstruction based on the patient’s clinical status and hemodynamic stability. Early diagnosis and prompt management of this condition are vital.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- ischemia
- vascular flow
- gastrectomy
- gastroesophageal reflux disease
- roux-en-y anastomosis
- fundoplication
- necrosis
- surgical procedures, operative
- venous insufficiency
- maize
- esophagus
- morbidity
- laparoscopic fundoplication
- esophagojejunostomy
- gastric bypass, roux-en-y
- blood supply, arterial
- collis gastroplasty
- compression



