-
PDF
- Split View
-
Views
-
Cite
Cite
Esmee W M Engelmann, Jelle J Posthuma, Lianne Scholten, Louise L Blankensteijn, Mireille B Boldewijn, Jan A H Gooszen, Gastrointestinal histoplasmosis mimicking peritonitis carcinomatosis: a rare case of an emergent surgical presentation of HIV de novo, Journal of Surgical Case Reports, Volume 2019, Issue 10, October 2019, rjz260, https://doi.org/10.1093/jscr/rjz260
Close - Share Icon Share
Abstract
Gastrointestinal perforation due to infection, including disseminated histoplasmosis, is a rare cause of the surgical acute abdomen, especially in an apparently healthy patient. We describe a rare case of gastrointestinal histoplasmosis-induced small intestine perforation as the first manifestation of acquired immune deficiency syndrome in a healthy patient. Remarkably, the disease mimicked peritonitis carcinomatosis during explorative laparoscopy.
INTRODUCTION
The acute abdomen accounts for approximately 7–10% of emergency hospital visits and up to 40% of surgical presentations [1, 2]. Gastrointestinal perforation is a common cause of acute abdomen and while some etiologies can be managed conservatively, others necessitate emergency surgery [3]. Frequent mechanisms of gastrointestinal perforation include extrinsic or intrinsic bowel obstruction (including tumor perforation), ischemia, foreign body perforation and in rare cases due to intra-abdominal infection [2]. This report presents a rare case of gastrointestinal perforation based on disseminated histoplasmosis infection.
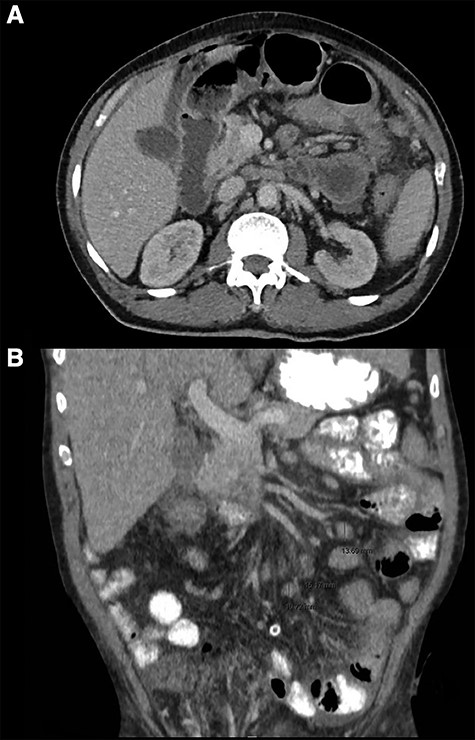
CT thorax abdomen, axial (A) and coronal (B) images. (A) Intra-abdominal free air and fluid, perihepatic, mesenteric and pelvic. (B) Mesenterial lymphadenopathy.
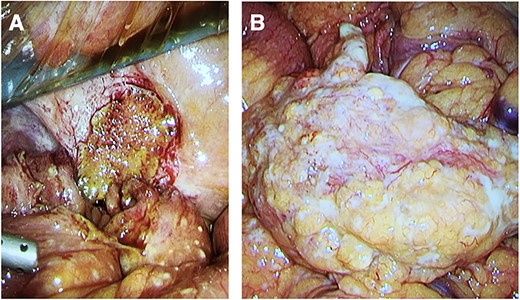
Intra-operative photos. (A) Site of the larger jejunal perforation, contained due to adhesion to the peritoneum. (B) Large studded white bowel mass with numerous peritoneal and omental depositions.
CASE REPORT
A 63-year-old male of Surinamese descent was admitted to the hospital with acute onset of abdominal pain, nausea and vomitus. There were no other bowel symptoms such as hematochezia, melena, fevers or diarrhea. Past medical history revealed herpes zoster ophthalmica and renal failure after postrenal obstruction due to benign prostate hyperplasia for which he underwent an uncomplicated transurethral resection of the prostate 6 months before. The patient suffered from weight loss in the past 6 months without a clear cause. On admission, the patient was hemodynamically stable with a mild tachycardia (112 beats per minute), normotensive (134/81 mmHg) and fever (39.5°C). Physical examination revealed a distended, hypertympanic and painful abdomen with a large right inguinal hernia without signs of incarceration. Laboratory tests showed an anemia (hemoglobin 6.1 mmol/l), normal white blood cell count (8.2 × 109/l) and mildly increased C-reactive protein (56 mg/l).
Computed tomography (CT)-imaging of the thorax and abdomen revealed intra-abdominal air and free fluid, suggestive of perforation of an unknown origin (Fig. 1A). Additionally, there were signs of pulmonary sarcoidosis and hilar and mesenteric lymphadenopathy.
Emergency laparoscopy was performed the same night. Intraoperative findings included fecal peritonitis and numerous diffuse white studs on the peritoneum, smaller intestine and mesentery, raising the suspicion for peritonitis carcinomatosis (Fig. 2). The right inguinal hernia was observed, containing non-incarcerated omentum and small bowel. The aspect of both liver and stomach was normal. Two small intestine perforations associated with the white lesions were found, for which two segments were resected and sent for pathological examination along with aspirated fluid. Primary extra-corporal anastomosis was performed by two stapled side-to-side enteroenterostomies.
Unexpectedly, histological examination was not consistent with malignancy but showed signs of yeast and chronic granulomatous inflammation with necrosis, most likely caused by histoplasmosis (Fig. 3). Differential diagnosis included tuberculosis and sarcoidosis. Patient history for such disease was negative; there were no pulmonary symptoms, and travel history in the last decade was negative. Given the pulmonary nodules on initial CT scan and negative sputum tests, a bronchoscopy with bronchoalveolar lavage was performed and Histoplasma capsulatum was found. This confirmed the diagnosis of disseminated histoplasmosis. Given that histoplasmosis rarely occurs in immunocompetent individuals, additional tests for acquired immune deficiency syndromes were performed, revealing a positive human immunodeficiency virus (HIV)-test. The viral load was very high (>10 000 000) with a low CD4 count (CD4% 5 [28–57], CD4 total 20 [560–1490 × 106/l]).
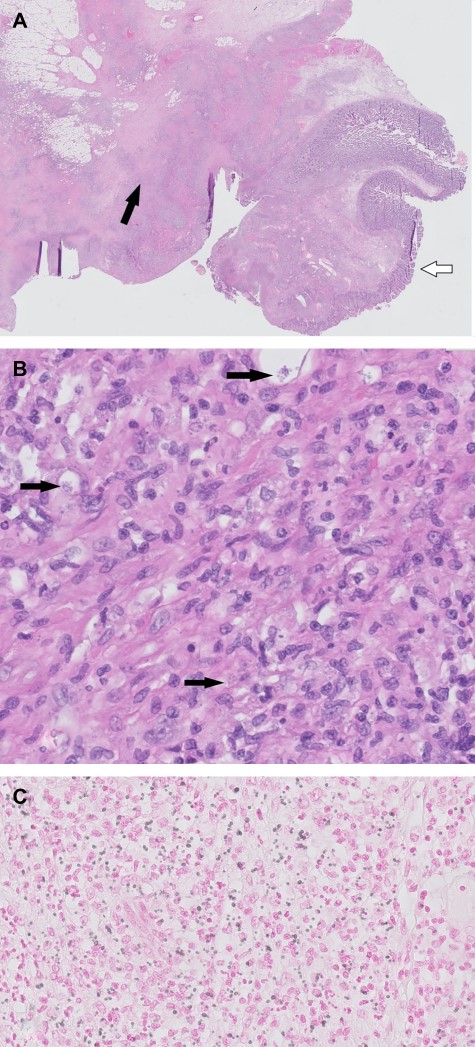
Histopathological images. (A) H&E overview duodenal resection specimen. White arrow: normal duodenal mucosa. Black arrow: inflammation with fibrosis and necrosis. (B) H&E 400×. Black arrows: histoplasmosis. (C) H&E 400×, Grocott staining histoplasmosis.
Postoperatively, the patient went to the intensive care unit for 2 days, where he developed pancytopenia and fever despite treatment with broad spectrum antibiotics. After the definitive pathological report, the patient was treated with co-trimoxazole as prophylaxis for Pneumocystis jiroveci pneumonia and intravenous liposomal amphotericin B (5 mg/kg 1dd) for histoplasmosis. Treatment with highly active antiretroviral therapy was not started directly due to risk of immune reconstitution inflammatory syndrome. Upon clinical deterioration in the immunocompromised patient, meropenem was started and the patient improved. Combination antiretroviral therapy was started and well tolerated. Amphotericin was switched to oral itraconazole. The patient was discharged home 4 weeks after surgery.
DISCUSSION
Histoplasma capsulatum is a dimorphic fungus endemic to Central and South America [4]. It is the most common organism in fungal respiratory infections [5]. In healthy hosts, histoplasmosis has a self-limiting course and patients are often asymptomatic. Disseminated histoplasmosis is a rare and life-threatening disease that is mostly found in immunocompromised patients (age extremes, hematologic cancers, use of immunosuppressive medication and untreated HIV with low CD4 levels [<200 cells/ml]) [4–6]. Interestingly, the disease may occur years after exposure, like our patient, who had not been in an endemic area for a decade. It is an acquired immunodeficiency syndrome (AIDS)-defining disease and mortality can be as high as 80% when left untreated [4, 7]. Radiological signs include diffuse nodular or interstitial pulmonary lesions and generalized lymphadenopathy. Pathological findings of gastrointestinal histoplasmosis include mucosal ulceration, diffuse lymphohistiocytic infiltration of the bowel wall (Fig. 3), submucosal nodules, polypoid lesions and obstructing masses [5, 8]. Enzyme immunoassay (EAI) is a rapid and reliable test for disseminated histoplasmosis by detection of the antigen in urine or serum with a sensitivity of 90% [4, 5]. It may also be used during follow-up to monitor therapy efficacy. The management of our patient with severe histoplasmosis is according to international guidelines: initial therapy with lipid formulation of amphotericin B and once patients improve clinically, this should be switched to itraconazole [4].
Although the majority of patients with disseminated histoplasmosis have gastrointestinal involvement, only 3–12% of the patients are clinically diagnosed after symptoms such as abdominal pain, intermittent diarrhea and weight loss [4, 5, 9]. In our case, systematic symptoms such as fever, night sweats and fatigue only manifested after surgery. The most frequently affected sites are the colon and distal ileum [6]. Bowel obstruction has been reported in a number of case reports and series. This is the fourth case of small bowel perforation due to abdominal histoplasmosis in history [6, 9, 10]. Physicians may consider histoplasmosis in the differential diagnosis of a patient with peritonitis of unknown origin [6].
Disseminated histoplasmosis is an AIDS-defining disease that can be difficult to diagnose for both surgeons and internal medicine physicians, due to its nonspecific presentation. In particular, perioperative similarity to peritonitis carcinomatosis can be misleading. Histopathology of surgical specimens and additional EAI are reliable diagnostic methods. Since antiretroviral and antifungal therapies have significantly improved the health of HIV patients, the greatest challenge is early diagnosis and recognition of surgical consequences of HIV and AIDS.
Conflict of interest statement
The authors declare that they have no conflicts of interest.



