-
PDF
- Split View
-
Views
-
Cite
Cite
Yu Huang, Masaaki Hidaka, Mitsuhisa Takatsuki, Akihiko Soyama, Tomohiko Adachi, Shinichiro Ono, Tota Kugiyama, Takanobu Hara, Satomi Okada, Tomoko Yoshimoto, Takashi Hamada, Susumu Eguchi, Surgical findings and technical knacks to performing living donor liver transplantation for hepatocellular carcinoma recurrence after carbon ion radiotherapy, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy228, https://doi.org/10.1093/jscr/rjy228
Close - Share Icon Share
Abstract
Although carbon-ion radiotherapy (CIRT) has been reported to achieve good local control of hepatocellular carcinoma (HCC), liver transplantation is still required in patients with tumor recurrence. However, few cases of living donor liver transplantation (LDLT) after curative CIRT for HCC has been reported. It would be of great interest to ascertain the true situation of the irradiated region as well as to clarify the surgical points. We herein report the surgical findings and our experience along with technical difficulties and knacks concerning two cases of LDLT for HCC after CIRT. Both patients suffered tumor recurrence after curative CIRT for HCC. Severe adhesions were found between the irradiated region and the surrounding tissues, which resulted in surgical difficulties. Histological findings showed severe tissue fibrosis in the CIRT area. We should pay attention to adhesions in the irradiated area caused by CIRT including the vascular reconstruction during surgery.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the second leading cause of cancer death worldwide and in less developed countries and the sixth leading cause of cancer death in more developed countries among men [1]. Carbon-ion radiotherapy (CIRT) has been developed as an effective treatment for HCC with cirrhosis [2]. The effectiveness was equivalent to the results of hepatectomy, independent of tumor location, and excellent local control of even tumors adjacent to the hepatic hilum can be obtained [3]. Furthermore, thus far, no death or liver failure has been reported. The local control rate at 5 years is over 80%, and the overall survival rate at 3 years is 50% [4].
We recently reported the first case of living donor liver transplantation (LDLT) after curative CIRT for HCC with portal vein invasion [5]. This report confirmed a pathological complete response of CIRT, although the surgical findings were not reported. LDLT for HCC recurrence after CIRT remains rare. Thus far, only two patients with HCC recurrence after CIRT have undergone LDLT at our hospital.
We herein report the surgical findings and our experience along with the technical difficulties and knacks associated with these two cases in order to provide suggestions regarding the management of similar patients.
CASE REPORT
Case 1
A 50-year-old woman with a diagnosis of HCC-related liver failure and hepatitis C virus (HCV) infection-related liver cirrhosis (LC) was referred to our hospital (Table 1). She was diagnosed with chronic hepatitis C in 2002 and received splenectomy due to portal hypertension in 2006. Interferon therapy was administered several times, and she achieved a sustained virological response in 2011.
| . | Case 1 . | Case 2 . |
|---|---|---|
| Character | 50-year-old female | 60-year-old male |
| Medical history |
|
|
| Diagnosis in Lt |
|
|
| Treatment |
|
|
| . | Case 1 . | Case 2 . |
|---|---|---|
| Character | 50-year-old female | 60-year-old male |
| Medical history |
|
|
| Diagnosis in Lt |
|
|
| Treatment |
|
|
SVR, sustained virologic response; CECT, contrast-enhanced computed tomography; PH, portal hypertension; PV, portal vein; LC, liver cirrhosis; HCC, hepatocellular carcinoma; CIRT, carbon-ion radiotherapy; MELD, Model for End-Stage Liver Disease; LDLT, living-donor liver transplantation; HCV, hepatitis C virus; FFP, fresh-frozen plasma; RBC, red blood cell; PC, platelet cell.
| . | Case 1 . | Case 2 . |
|---|---|---|
| Character | 50-year-old female | 60-year-old male |
| Medical history |
|
|
| Diagnosis in Lt |
|
|
| Treatment |
|
|
| . | Case 1 . | Case 2 . |
|---|---|---|
| Character | 50-year-old female | 60-year-old male |
| Medical history |
|
|
| Diagnosis in Lt |
|
|
| Treatment |
|
|
SVR, sustained virologic response; CECT, contrast-enhanced computed tomography; PH, portal hypertension; PV, portal vein; LC, liver cirrhosis; HCC, hepatocellular carcinoma; CIRT, carbon-ion radiotherapy; MELD, Model for End-Stage Liver Disease; LDLT, living-donor liver transplantation; HCV, hepatitis C virus; FFP, fresh-frozen plasma; RBC, red blood cell; PC, platelet cell.
In 2014, she was administered CIRT with a total of 60 Gy (relative biological effectiveness) given in four fractions because of rapidly enlarged tumor with segmental portal vein invasion, as shown on contrast-enhanced computed tomography. Before admission, CT revealed a new recurrence tumor in the lateral segment of the left lobe (Fig. 1A). The irregular surface and unsmooth liver edge also showed LC. Furthermore, severe atrophy was obvious at the CIRT area (Fig. 1A). Because of the new lesion in the left lobe located close to the heart and the underlying liver failure with C-P grade B (9 points), LDLT was performed with an extended left lobe graft from her son in 2015. During surgery, strong adhesion between the thoracic diaphragm and the irradiated lesion in Segment 7 was observed, which resulted in difficulty with adhesiolysis (Fig. 1B). If we preserve the diaphragm, it might be injured irradiated liver during adhesiolysis. We intensively choose a part of diaphragm resection with liver, also protected inferior vena cava (IVC) from injury during resection of diaphragm. We checked where the IVC existed in the thoracic cavity and preserved the IVC above the liver, encircling the right hepatic vein (RHV) after mobilization of the liver. The diaphragm was then repaired directly by suturing. After reconstruction of the hepatic vein (HV), portal vein (PV), hepatic artery and bile duct, we successfully finished the operation. The surgery took over 11 h to complete, and the total amount of blood lost was 2500 g with 10 units of fresh-frozen plasma transfusion (FFP). The specimens showed no HCC recurrence in the CIRT area but did show severe fibrosis (Fig. 2).
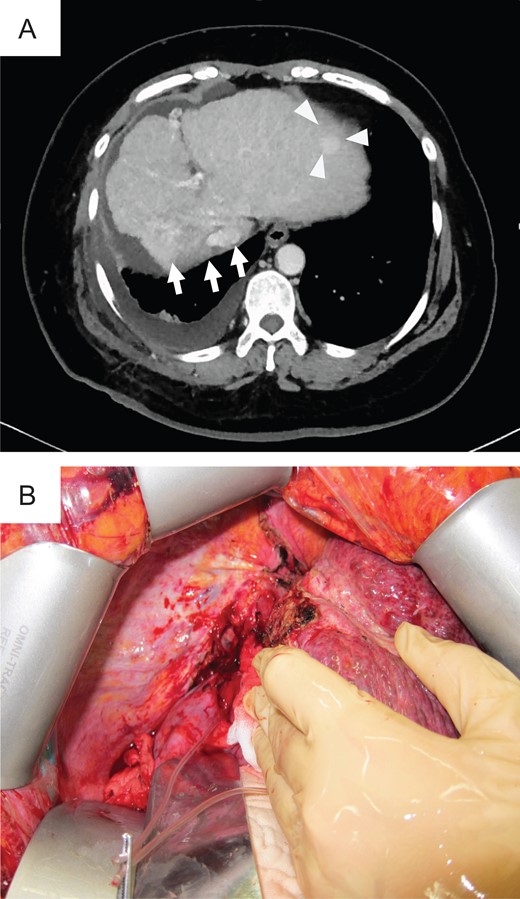
Preoperative photograph and intraoperative images of case 1. (A) Liver atrophy was noted in the CIRT area (arrow), and a new lesion (triangle) was noted in the left lobe on preoperative CT. (B) Intraoperative imaging showed strong adhesion between the thoracic diaphragm and the CIRT area.
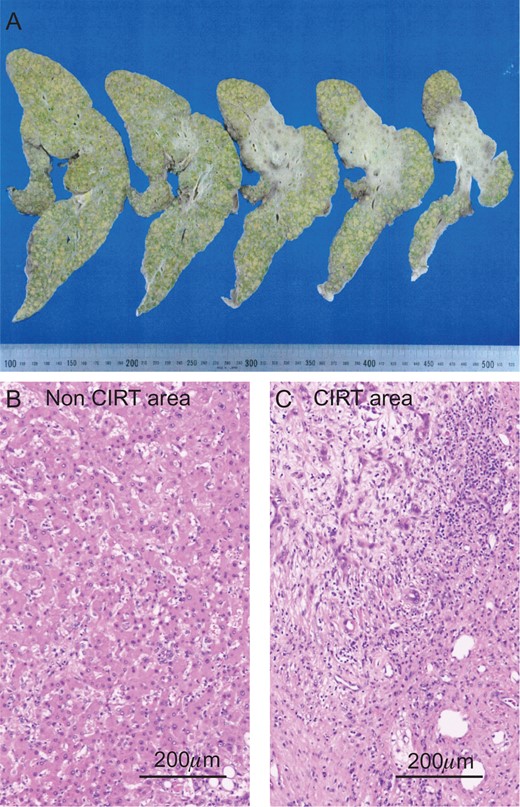
The liver specimen of case 1. (A) The irradiated area showed an off-white color in the liver (dotted line area). H&E staining revealed normal liver in the non-CIRT area (B) and fibrotic liver in the CIRT area (C).
Case 2
The patient was a 60-year-old man who had been diagnosed with HCV-related LC and HCC (Table 1). In 2014, this patient underwent CIRT with a total of 60 Gy (relative biological effectiveness) given in four fractions for HCC in S4 and was in complete remission (CR). In 2015, multiple HCC was observed on CT (Fig. 3A). After treatment with TACE, he met the Milan criteria and underwent LT. Preoperative magnetic resonance imaging with gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid (EOB-MRI) revealed atrophy in the CIRT area and hypertrophy in the left liver (Fig. 3B).
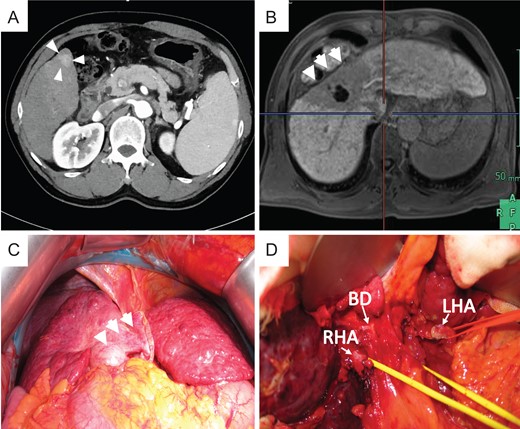
Preoperative photograph and intraoperative images of case 2. (A) New lesion in S6 on preoperative CT. Atrophy was noted in the CIRT area, and hypertrophy was noted in the left liver on preoperative EOB-MRI (B) and intraoperative images (C). (D) Severe tissue adhesion on the hepatic hilar resulted in difficulties during adhesiolysis.
During surgery, strong atrophy of the liver parenchyma was found in the irradiated area (Fig. 3C). Tissues adjacent to the irradiated area were also strongly adhered. During dissection of the right side in the liver hilum, the right hepatic artery (RHA) was injured due to inflexible conglutination (Fig. 3D). We sutured and closed the bleeding point of the RHA and decided to separate the RHA without detachment between the RHA and bile duct. Carefully, the liver was retrieved after we successfully secured the common bile duct, left hepatic artery (LHA), PV and HV. This case is also transplanted with left lobe graft. We reconstructed the middle hepatic vein and left hepatic vein (MHV–LHV) in the recipient to the MHV–LHV in the donor by continuous sutures with 4-0 Prolene, the left branch of the PV in the recipient to the left branch of the PV in the donor by continuous sutures with 5-0 Prolene, and the LHA in the recipient to the LHA in the donor by interrupted sutures with 8-0 Prolene using microscopy.
After reconstruction of the LHA, we found that the graft backflow from the middle hepatic artery (MHA) of the liver graft was weak. Therefore, we decided that reconstruction of the MHA was necessary. As the recipient’s RHA was not suitable for reconstruction due to CIRT, we chose to use the right gastroepiploic artery (RGEA) in the recipient. We performed reconstruction between the RGEA in the recipient to the MHA in the donor by interrupted sutures with 8-0 Prolene using microscopy.
The surgery took over 14 h to complete, and the total amount of blood lost was 4400 g, with 6 U of red blood cell (RBCs), 25 U of FFP and 10 U of platelet (PC) transfusion. The histological specimens showed severe fibrosis along with heavy cirrhosis and strong atrophy but no HCC recurrence in the CIRT area (Fig. 4).
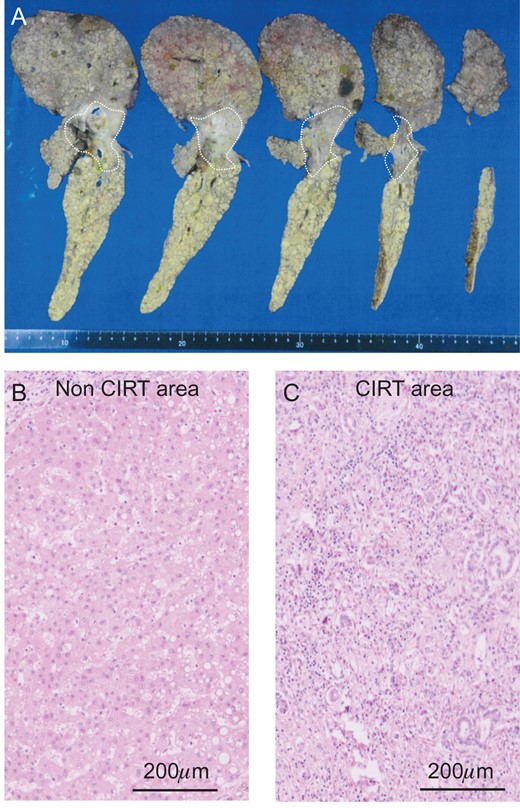
The liver specimen of case 2. (A) The irradiated area showed an off-white color in the liver (dotted line area). H&E staining revealed normal liver in the non-CIRT area (B) and fibrotic liver in the CIRT area (C).
DISCUSSION
CIRT has been reported to be safe and effective for treating HCC, and it causes only minor liver damage [2]. As shown in our two cases, the region irradiated with CIRT showed atrophy, and the non-irradiated region demonstrated compensatory enlargement. This explains why CIRT causes relatively little liver damage with a retained liver function after CIRT [6]. It was suspected that the compensatory enlargement had a contributory role in the retention of the hepatic function. However, both of the cases presented here showed recurrence of HCC in the non-irradiated region and usually accomplished with decompensated liver function, which is interesting that time interval of recurrence in both two cases were nearly 1 year after CIRT. This may serve as a remained to perform a 1-year surveillance period after CIRT for HCC.
CIRT causes severe fibrosis in the irradiated region and adhesion between the irradiated area and surrounding tissues, which might be accompanied by a high risk of difficulty in surgery and increased risk of complications. In case 1, strong adhesion between the thoracic diaphragm and S7 resulted in partial resection of the thoracic diaphragm to avoid liver injury during detachment between the diaphragm and liver. After resecting part of diaphragm, we must pay close attention to avoid injuring the IVC. Following CIRT for HCC around the IVC, it might be difficult to separate the IVC and diaphragm, unless IVC is suddenly injured, we choose to open thoracic cavity, check the IVC under right. In case 2, tissues adjacent to the hepatic hilum resulted in separation of the RHA during adhesiolysis, which rendered non-anatomical reconstruction of the HA necessary and increased the difficulty of vessel reconstruction in LDLT. CIRT caused vessels and tissues adhered to around. We recommend that radiologists carefully plan CIRT for HCC near the liver hilum in patients with LC who may need to undergo LT in the future. In both of our cases, strong adhesion due to CIRT was difficult to predict based on preoperative photographs; such images will need to be carefully evaluated before surgery and meticulously administered during operation.
The two present cases showed adhesion and fibrosis characteristics of CIRT. Surgically, we should pay attention to adhesion in the irradiated area caused by CIRT including the vascular reconstruction during. We believe that our surgical experience will be beneficial for managing other LDLT cases with HCC after CIRT. More knowledge must be gained regarding the hepatic histopathological features after CIRT for HCC in order to improve the understanding of the biological specificity of CIRT for HCC and possibly optimize the treatment.
CONFLICT OF INTEREST STATEMENT
Non declared.
FUNDING
None.



