-
PDF
- Split View
-
Views
-
Cite
Cite
Amit K Mahajan, Mia Newkirk, Carolyn Rosner, Sandeep J Khandhar, Successful endobronchial treatment of a non-healing tracheoesophageal fistula from a previous histoplasmosis capsulatum infection using decellularized porcine urinary bladder matrix, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy187, https://doi.org/10.1093/jscr/rjy187
Close - Share Icon Share
Abstract
Tracheoesophageal fistulas (TEF) are pathologic communications between the esophagus and the trachea or bronchi. The development of a TEF can result from malignant or benign etiologies. A common approach for the treatment of TEFs is the placement of endobronchial and esophageal stents to facilitate healing of the communication. This case report describes the successful treatment of a TEF resulting from calcified mediastinal lymphadenopathy due to a previous Histoplasmosis capsulatum infection. In addition to placement of endobronchial and esophageal stents, the non-healing TEF was treated with ACell (Gentrix®) decellularized porcine urinary bladder matrix to facilitate complete closure of the fistulous tract.
INTRODUCTION
Tracheoesophageal fistulas (TEF) are pathologic communications between the esophagus and the trachea or bronchi [1]. The development of a TEF can result from malignant or benign etiologies and can be life-threatening. Tumor invasion of esophageal malignancies and complications from treatment of these malignancies are the most common causes for the development of TEFs [2]. Benign etiologies for TEFs include complications from tracheal or esophageal surgery along with granulomatous mediastinal infections, iatrogenic injuries and caustic ingestions [3, 4].
The management of TEFs ranges from endoscopic stent placement to invasive surgical repair. Surgical repair of TEFs is associated with high morbidity and mortality. Additionally, stenting of the airway and esophagus is often poorly tolerated by patients. This report describes successful treatment of a non-healing TEF stemming from Histoplasmosis capsulatum despite airway and esophageal stenting using a decellularized porcine urinary bladder matrix.
PATIENT PRESENTATION
The patient was a 66-year-old white female with history of Histoplasmosis capsulatum infection and diabetes who presented as an outpatient with hemoptysis for 6 weeks. The patient was a lifelong non-smoker. A computer tomography (CT) scan of the chest revealed a calcified subcarinal lymph node along with an obstructing endobronchial lesion on the medial wall of the right mainstem bronchus (Fig. 1A). A rigid bronchoscopy was performed revealing an endobronchial mass causing a 50% obstruction of the right mainstem bronchus (Fig. 1B). The cryotherapy probe was used for mass excision and a full mediastinal staging was performed using endobronchial ultrasound (EBUS) bronchoscopy. The right mainstem bronchus was recanalized with return to 100% patency. Histology from the mass revealed an inflammatory polyp. The etiology of the inflammatory polyp was presumed to arise from inflammation stemming from the calcified station 7 lymph node abutting the medial wall of the right mainstem bronchus.
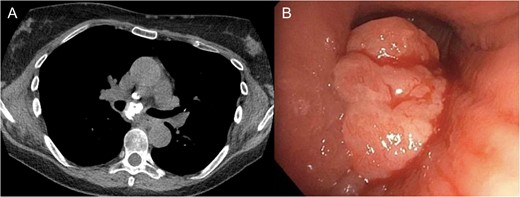
(A) Calcified subcarinal lymph node. (B) Obstructing endobronchial lesion on the medial wall of the right mainstem bronchus.
One-year after her original presentation, the patient presented to the emergency room with worsening cough and choking with oral intake. A CT scan of chest revealed a fistula between the right mainstem bronchus and esophagus (Fig. 2A). The patient underwent rigid bronchoscopy revealing a 2-cm defect on the medial wall of the right mainstem bronchus, ~1-cm from the main carina (Fig. 2B). A CT esophagram confirmed extravasation of oral contrast into the tracheobronchial tree (Fig. 3).
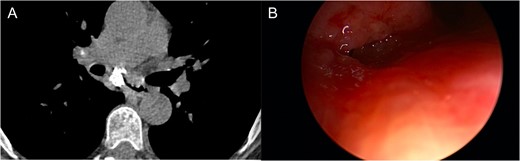
(A) CT scan revealing fistula between the right mainstem bronchus and esophagus. (B) Endobronchial view visualizing right mainstem defect.
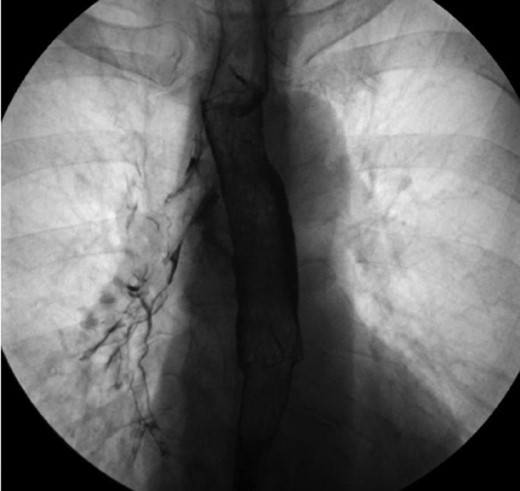
Esophagram revealing extravasation into the tracheobronchial tree.
Based on the location and size of the defect, a 16 mm × 13 mm × 13 mm Bryan Dumon™ silicone Y-stent was successfully deployed to completely cover the airway defect (Fig. 4). An esophagogastroduodenoscopy (EGD) was then performed that revealed a small mucosal defect in the mid-esophagus. The defect was covered with a 23 mm × 150 mm Merit Endotek™ EndoMAXX fully covered metallic esophageal stent. Following both procedures, the patient’s symptoms resolved and she was discharge home with close follow-up.
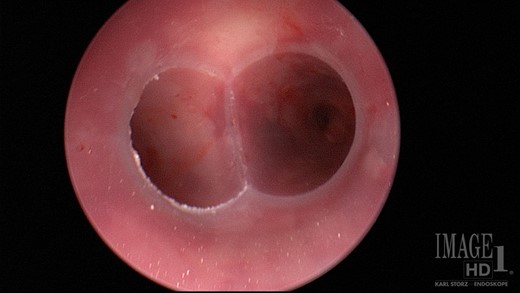
A rigid bronchoscopy was performed 8 weeks after the original endobronchial stent placement. The flexible bronchoscope was passed around the Y-stent to visualize the medial wall of the right mainstem bronchus. The site of the fistulous communication had improved ~80%, but a small residual defect was still present. Despite improvement in the size of the fistulous communication, the defect appeared be quite deep and did not show signs of apposition of the opposing walls of the fistula that would aid in healing. Additionally, there was concern that the Y-stent may have also have been contributing to poor healing due to persistent rubbing at the defect with coughing. In order to aid in granulation formation and to facilitate healing, four layers of ACell® decellularized porcine urinary bladder matrix were placed over the defect bronchoscopically (Fig. 5). In order to place the Acell® matrix at the site of the defect, the Olympus Medical 6.0 mm therapeutic bronchoscope was passed around the tracheal limb of the Y-stent. The bronchoscope was advanced between the Y-stent and posterior wall of the trachea down to the main carina and to the right medial wall defect. Under direct visualization, four strips of the ACell® matrix were placed over the defect using Olympus Medical flexible biopsy forceps. Once the bronchoscope was removed, the ACell® matrix was held in place over the defect by the Y-stent. Due to the semi-opaque nature of the Y-stent, confirmation that the ACell® matrix was in the correct location was possible as it could be visualized when the bronchoscope was positioned within the Y-stent at the main carina.
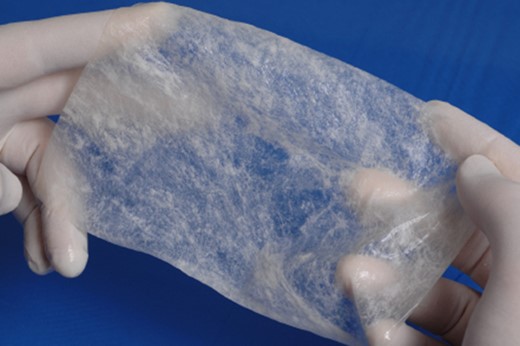
ACell® decellularized porcine urinary bladder matrix over the defect to aid in granulation formation and to facilitate healing.
Ten days after the ACell® matrix placement, a rigid bronchoscope was again performed. Complete resolution of the defect had occurred. The Y-stent and the esophageal stent were removed the following week with resolution of symptoms. Repeat imaging following removal of the stents, including a non-contrast CT of the chest and a CT esophagram, demonstrated resolution of the fistulous communication. Repeat bronchoscopy was performed 10 months after stent removal with no evidence of fistula recurrence and minimal persistent inflammation (Fig. 6).
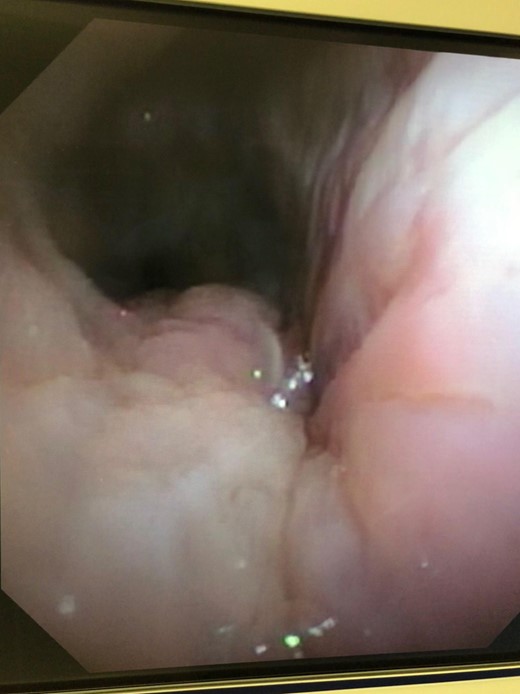
DISCUSSION
The development of a TEF resulting from calcified mediastinal lymph nodes secondary to Histoplasmosis capsulatum is rare, with few studies describing the phenomenon [5]. Surgical intervention for repair of a TEF secondary to Histoplasmosis capsulatum infection may pose a more significant challenge due to mediastinal fibrosis. Due to the extensive mediastinal calcification, surgical intervention was deemed to be technically difficult and may be associated with significant morbidity and mortality.
Esophageal and/or airway stenting has gained favor compared to surgery to seal TEFs and prevent the leakage of liquid or gas. Airway stent placement with or without esophageal stenting has been observed in a number of small studies to improve clinical symptoms and has led to fistula resolution in some cases [6]. Additionally, the scarring properties of stenting can be utilized to facilitate closure of a TEF and, therefore, has been a preferred therapeutic approach [7]. Although treatment of a TEF using endobronchial and esophageal stenting may be difficult for patients to tolerate, it provides an excellent initial treatment option.
The ACell® matrix is a decellularized porcine urinary bladder matrix used to facilitate naturally adaptive or accommodative immune response, which is conducive for wound healing and three-dimensional growth of various cell types [8]. The porcine bladder serves as scaffolding for tissue growth and healing. A number of studies have utilized ACell® technology to aid in healing of diabetic foot ulcers and non-healing wounds. ACell® matrix is not indicated for use in TEFs and therefore this device was used in an off-label fashion. Although the fistulous communication in this case had partially healed with airway and esophageal stenting, complete closure of the fistula was not achieved after eight weeks. The ACell® matrix was placed over the residual area non-healing TEF and was held in place by the Y-stent for 10 days. Prior to removal of the stent, visualization of the defect revealed complete closure of the TEF. Additionally, CT imaging of the chest following Y-stent removal revealed a significant layer of scar tissue between the subcarinal lymph node and right mainstem bronchus, which will hopefully prevent recurrence of the fistula. Based on the success of ACell® matrix to facilitate healing, further studies are warranted to explore additional uses for this technology within the airway.
This case features the novel use of ACell® decellularized porcine urinary bladder matrix to facilitate resolution of a non-healing TEF in conjunction with airway and esophageal stenting. The significant morbidity associated with TEFs requires further development of expeditious and sustainable treatment options such as ACell® therapy that appear to be as effective as surgery without the associated risk.
CONFLICT OF INTEREST STATEMENT
We declare that we have no conflicts of interest
AUTHOR CONTRIBUTION
Each author has contributed equally to this article.
REFERENCES
Author notes
This article has not been submitted for publication in any other journals.



