-
PDF
- Split View
-
Views
-
Cite
Cite
Raelina S Howell, James A Rice, Kristin Sticco, Virginia Donovan, Michael Castellano, Brian Gillette, Scott Gorenstein, An unusual presentation of Merkel cell carcinoma: a case report, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy185, https://doi.org/10.1093/jscr/rjy185
Close - Share Icon Share
Abstract
Merkel cell carcinoma (MCC) is a rare, aggressive carcinoma that usually arises in sun-exposed regions. MCC is a primary neuroendocrine tumor that arises in the skin. This report describes an unusual case of MCC on the buttocks that was treated with excision, radiation and chemotherapy. Physicians should consider MCC as a differential diagnosis when encountering a rapidly growing, painless lesion. Early diagnosis and treatment may improve patient survival rates.
INTRODUCTION
Merkel cell carcinoma (MCC) is a rare, primary neuroendocrine cutaneous neoplasm that usually appears in elderly and/or immunosuppressed males. MCC typically presents as a painless, solitary lesion on sun-exposed regions of the body (e.g. head, neck, extremities) with initial indolent growth. However, the course of MCC is aggressive with nodal invasion, distant metastasis and high recurrence rates [1]. This report describes an interesting case of MCC discovered on the left buttocks.
CASE REPORT
A 68-year-old Caucasian male presented to the emergency department (ED) with a painless, draining left buttock lesion. He reported that the wound had originated as a pimple a few months earlier, but had been growing over the last three weeks with foul-smelling discharge. There was no history of trauma to the area. His past medical history included a deep vein thrombosis/pulmonary embolism 20 years prior for which he was on warfarin, hyperlipidemia and tonsillectomy. On physical exam, the patient’s vitals were significant for blood pressure elevated to 153/87. On the left gluteus, a 10 × 10 cm2 non-tender lesion was noted with malodorous discharge. Labs were only significant for a therapeutic international normalized ratio of 3.07. A computed tomography (CT) of the pelvis was read as having a 9 × 10.8 × 4.2 cm3 left gluteal abscess and bulky left inguinal lymphadenopathy (Fig. 1). The patient was admitted, started on intravenous antibiotics and taken to the operating room (OR) for unroofing, debridement and incisional biopsy (Fig. 2). Pathology revealed ulcerated, necrotic skin fragments with MCC and cultures grew Enterobacter cloacae, Haemophilus parainfluenzae, Peptostreptococcus anaerobius, Peptostreptococcus asaccharolyticus. Antibiotics were de-escalated and the patient was discharged with sodium hypochlorite wound dressing changes. A chest CT was performed and showed no evidence of distant metastasis. One month later, the patient then underwent complete operative excision of the lesion down to the gluteus fascia with 2 cm margins and left groin lymph node dissection with lymphadenectomy. The resulting 13 × 15 cm2 defect was closed with a combination of fasciocutaneous advancement flaps and split-thickness skin grafting (Fig. 3). Pathological analysis showed a completely excised 11.6 cm ulcerated MCC lesion with negative margins and positive lymphovascular invasion (2/2 positive lymph nodes)—stage pT3 pN1b (Fig. 4).
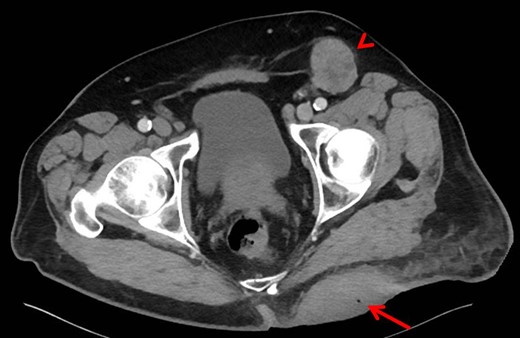
Computed tomography of pelvis. The left gluteal lesion with a foci of air (arrow) signifying possible infected versus necrotic material. Left inguinal lymphadenopathy also demonstrated (arrowhead).
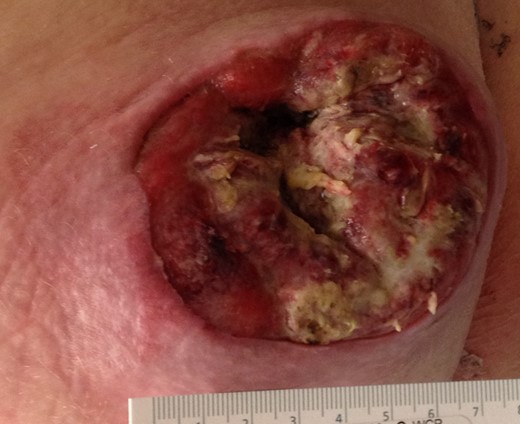
Left buttock lesion. Appearance of the wound following unroofing, drainage and incisional biopsy.
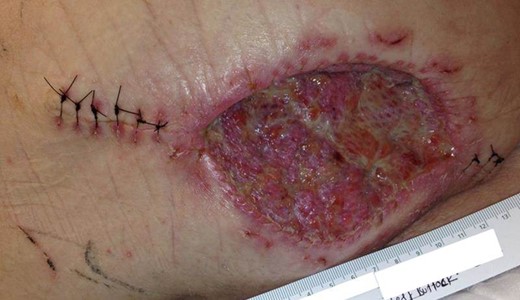
Postsurgical excision. Appearance of the excision site on postoperative day seven following partial closure with fasciocutaneous advancement flap and split-thickness skin grafting.
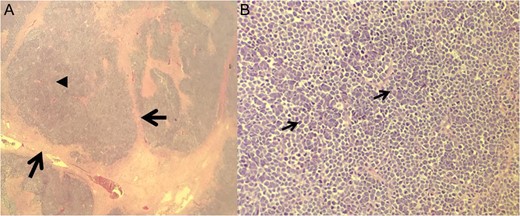
Histological results of excision. (A) ×10 Fibrotic cords (arrows) surrounding the MCC cells and central tumor necrosis (arrowhead). (B) ×40 Bland cell morphology (arrows) with a mix of fine, bland and powdery chromatin.
A hematology oncology consult was appreciated and per their recommendation the patient completed a course of 6120 cGy pelvic to buttock radiation over 2 months. Hyperbaric oxygen therapy, an established treatment for non-healing wounds within a radiated field [2, 3], was offered, but the patient declined. The buttock wound was noted to be healed at the 5-month postoperative follow up (Fig. 5). A follow-up CT of the chest, abdomen and pelvis displayed increasing supraclavicular, mediastinal and retroperitoneal lymphadenopathy. The patient elected to follow up at a specialized clinical trials center and received pembrolizumab.
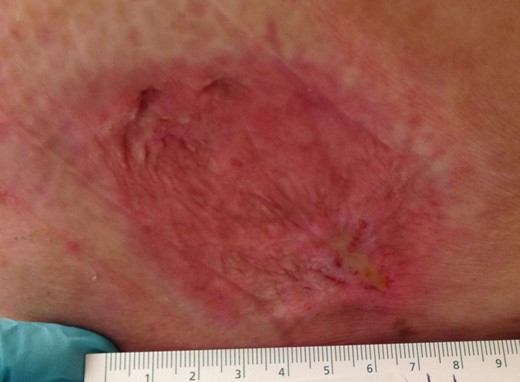
Healed wound. Appearance of the left buttock wound five months following complete MCC excision.
DISCUSSION
MCC is a primary anaplastic carcinoma of the skin that can arise in the dermoepidermal junction [4]. Merkel cells are dermal sensory neuroendocrine and mechanoreceptors that are located at the stratum basale epidermal layer [1]. Numerous etiological factors contribute to MCC development such as exposure to UV radiation, infection with Merkel cell polyomavirus (MCPyV) and chronic immunosuppression. MCPyV is a human polyomavirus that is linked with MCC in up to 80% of cases [1]. More than 9 out of 10 people diagnosed with MCC are Caucasian and above the age of 50 [5]. MCC is most commonly found in sun-exposed regions of the body—55% head/neck versus 5% buttock. The estimated 5-year survival rates for MCC are based on the stage of the disease and range from 18 to 80% [5, 6].
MCC can present clinically as a painless, firm, bluish-red, rapidly growing nodule. Overlying skin at the site of exposure may exhibit acneiform, telangiectasia or ulcerative characteristics [1]. Histologically, MCC appears as small, round, blue cells with sparse cytoplasm, medium to large-sized hyperchromatic nuclei, multiple small nucleoli, delicately granular chromatin, abundant mitoses and numerous apoptotic figures [1]. MCC is occasionally mistaken for other histologically related cutaneous tumors such as small cell lung carcinoma or extra skeletal primitive neuroendocrine tumors [1]. Diagnosis of MCC is confirmed by hybrid light microscopy, electron microscopy and immunohistochemistry [1].
Locoregional treatments include wide excision, adjuvant radiation therapy, and if applicable, regional lymph node excision. The common sites of metastasis are the dermis, liver, lungs, bones, brain and lymph nodes [1]. Metastasis detection with MCC can be done via CT, MRI, PET scan and octreotide scintigraphy [1]. In addition, radiotherapy to the primary site with or without inguinal nodes with palliative intent has been shown to significantly improve relapse-free survival rates. This patient’s pathology results in a stage 3B MCC with a 25% 5-year survival rate. It is important for wound care physicians to be aware of this uncommon presentation of MCC, and there should be an index of suspicion for MCC in a rapidly growing painless lesion.
Currently, no curative treatments have been established for metastatic MCC. The role of chemotherapy is unclear and it is currently reserved for advanced-stage MCC and palliative therapy. Further studies are needed to assess the optimal combination of surgical resection, radiation and chemotherapy to improve disease-free survival.
CONCLUSION
MCC is a rare, aggressive carcinoma that usually arises in sun-exposed regions. Physicians should consider MCC as a differential diagnosis when encountering a rapidly growing, painless lesion. Early diagnosis and treatment may improve patient survival rates. However, due to the rarity of MCC, further studies are needed to develop treatment protocols for metastatic disease.
ACKNOWLEDGMENTS
We would like to thank NYU Winthrop Hospital and the NYU Winthrop Wound Care team for their support.
CONFLICTS OF INTEREST
The authors have no conflicts of interests or disclosures.



