-
PDF
- Split View
-
Views
-
Cite
Cite
Kayla M Humenansky, Raj Gulati, Small bowel gastrointestinal stromal tumor disguised as an adnexal mass: a source for midgut volvulus, Journal of Surgical Case Reports, Volume 2018, Issue 7, July 2018, rjy157, https://doi.org/10.1093/jscr/rjy157
Close - Share Icon Share
Abstract
Background: The abdominal cavity has an infinite number of potential pathologies and gynecologic pathology is often intertwined with intestinal disease. Case presentation: A 74-year-old female believed to have an adnexal mass on prior imaging presented with small bowel obstruction, which failed to resolve with non-operative management. Given her suspected adnexal mass, multidisciplinary operative intervention was arranged. She was found to have a large, extraluminal mass on her small intestines; serving as the lead point for her midgut volvulus and resultant small bowel obstruction. Conclusion: Physical exam and radiographic discordance should prompt consideration of alternative diagnoses. Making the appropriate initial diagnosis is key in correct patient management; however, this is not always possible and appropriate pre-operative planning should be arranged for best patient outcomes.
INTRODUCTION
Gastrointestinal stromal tumors (GIST) are rare mesenchymal tumors originating from the interstitial cells of Cajal, an intestinal pacemaker cell [1–4]. Accounting for <1% of all gastrointestinal tumors, they most commonly arise from the muscularis propria and tend to grow extramurally [3, 5]. They can be benign or malignant. Most GIST occur within the stomach; only 30% occurring within the small intestine [2, 3, 6]. Almost all GIST occur from mutations in the c-KIT oncogene, which codes for the expression of CD117 [3, 4]. They vary in presentation and clinical course ranging from vague abdominal pain to large tumors with necrosis or hemorrhage and even widespread metastasis [3, 4, 7]. With new advances in the medical treatment of GIST, the long-term prognosis has improved; however, surgical resection remains the standard of care [4]. We present a case of a midgut volvulus secondary to a GIST previously believed to be an ovarian mass.
CASE REPORT
A 74-year-old G3P3003, postmenopausal female with an incidental 7.6 cm lobulated pelvic mass seen on prior computed tomography (CT) for flank pain. Follow-up ultrasound (US) was consistent with a 5.9 × 3.4 × 2.9 cm3 uterus with 4–5 mm endometrium and 7.5 × 4.9 × 5.5 cm3 hypoechoic solid mass on the posterior aspect of the uterus with significant internal vascular flow (Fig. 1). The mass was noted to be separate from the uterus without evidence of free fluid or ascites; however, a normal right ovary was unable to be identified. No tumor markers were obtained at that time; however, she was counseled about her pelvic mass and referred to gynecologic oncology for further management. At that time, she denied any history of unintentional weight loss, fatigue, postmenopausal bleeding, pelvic pressure or pain, changes in diet, bloating or changes in bowel or urinary habits.
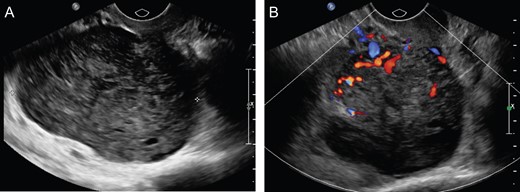
(A) Uterus measuring 5.9 × 3.4 × 2.9 cm3 with heterogeneously hypoechoic solid mass, measuring 5.5 × 4.9 × 7.5 cm3 right aspect of the uterus. (B) Color Doppler flow analysis with significant internal vascular.
While awaiting evaluation by gynecologic oncology, she presented acutely with nausea, vomiting and abdominal pain. At that time, she was found to have a small bowel obstruction (SBO) on CT imaging with concern for a transition point near her previously visualized pelvic mass (Fig. 2). She was given a trial of non-operative management, including nothing by mouth and nasogastric decompression; however, given her persistent abdominal pain and high nasogastric tube (NGT) output, it was thought best to proceed with operative intervention.
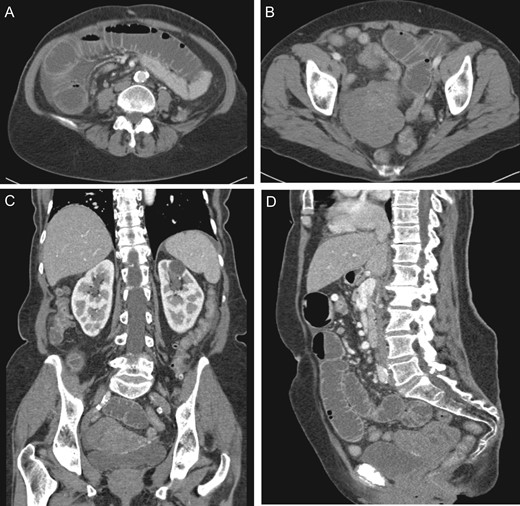
(A) Dilated small intestine with small amount of ascites. (B) Large heterogeneous pelvic mass. (C) Coronal view pelvic mass (arrow) with compression of bladder (arrow head). (D) Sagittal view pelvic mass.
After discussion with gynecology and gynecologic oncology, she underwent an exploratory laparotomy. Upon entry into the abdomen, distended and congested small intestine was visualized without apparent ischemic changes. The small bowel was twisted about the superior mesenteric artery axis (SMA) and tethered toward the pelvis. The small bowel was then eviscerated to reveal a large, pedunculated 9 × 7 × 8 cm3 mass, originating from the antimesenteric side of the small intestine and located 162 cm from the ileocecal valve. The small intestine was noted to have volvulized around this pedunculated mass. It was immediately detorsed and normal peristaltic function returned within minutes. Starting with the decompressed small intestine, the bowel was run from the ileocecal valve to the ligament of Treitz without any other abnormalities noted. A segmental small bowel resection was then performed to include the mass and associated mesentery. A total of 14 cm of bowel was resected (Fig. 3). The remainder of the abdomen was inspected and noted to be free of pathology, particularly the uterus, fallopian tubes and ovaries. Pathology demonstrated evidence of a serosal spindle-cell neoplasm, four mitotic figures/5 mm2 and immunohistochemistry strongly positive for CD117 and DOG1. No lymph node metastasis was identified. These findings were consistent with a low-grade GIST. Patient did well post-operatively and was discharged home on post-operative Day 6.
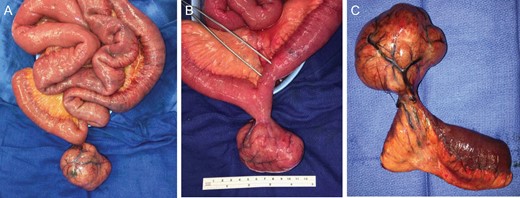
(A) Eviscerated small intestine with pedunculated mass. (B) Pedunculated mass with forceps indicating site of volvulus resulting in luminal narrowing from compromised blood flow. (C) Small bowel segmental resection.
DISCUSSION
GIST are rare mesenchymal tumors, with an estimated incidence of <1% per year [1]. Most GIST occur from mutations in the c-KIT oncogene, which codes for the expression of CD117 thus allowing them to be more easily identified on immunohistochemistry [3]. Given their ability to reach large sizes and typical extraluminal growth pattern, they can serve as the source for other intra-abdominal pathologies. The average size is ~5 cm when diagnosed clinically [3, 5, 6]. While our patient had the appropriate work-up, including pelvic US and CT scan, a definitive diagnosis remained unclear. GIST have a wide range of imaging characteristics and can appear as hypoenhancing, isoenhancing and hyperenhancing tumors, making them difficulty to decisively diagnose through imaging [8].
Given the mobility of the small intestine, small bowel GISTs may be hard to locate radiographically. At large size, they have a tendency to migrate toward the dependent portions of the abdomen putting them in close proximity to the pelvic organs. This makes it difficult to determine the exact etiology of the mass. Although gynecologic malignancy is always a concern with identification of a pelvic mass, one must remember the differential diagnosis is ostensibly infinite. If symptoms do not correlate with imaging, suspicion should be raised and alternative diagnoses other than gynecologic tumor must be considered. Operative intervention should be arranged accordingly, as complete and timely surgical resection offers the best long-term survival.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- diagnostic radiologic examination
- small bowel obstruction
- physical examination
- adnexal mass
- differential diagnosis
- intestinal diseases
- intestine, small
- patient care management
- diagnosis
- diagnostic imaging
- pathology
- gastrointestinal stromal tumor
- malrotation, congenital
- Abdominal cavity
- patient-focused outcomes



