-
PDF
- Split View
-
Views
-
Cite
Cite
Sami Khairy, Reham Khubrani, Sadeq Al-Dandan, Abdullah Alobaid, Thalamic germinoma: a challenging diagnosis, case report and literature review, Journal of Surgical Case Reports, Volume 2018, Issue 7, July 2018, rjy154, https://doi.org/10.1093/jscr/rjy154
Close - Share Icon Share
Abstract
The thalamus and basal ganglia are unusual locations for an intracranial germ cell tumors. We are reporting a rare case of thalamic germinoma in an 18-year-old male. Challenging presentation, radiological appearance and pathological finding after surgical intervention delayed the diagnosis and treatment. Also, we are providing an extensive literature review. Diagnosis of thalamic germinoma is challenging because of non-specific symptoms, rare location and inconclusive radiological findings. An early tissue diagnosis associated with good outcome.
INTRODUCTION
Germ cell tumors (GTC) represent only 0.5–2% of all primary intracranial tumors. The most frequent type of these GTC is the germinoma. Germinomas have a predilection to occur in the pineal region and the hypothalamic area [1]. Diagnosis of these lesions without a sufficient pathological sample is challenging [2].
We are reporting a rare case of thalamic germinoma with a challenging presentation and diagnosis.
CASE DESCRIPTION
An 18-year-old male without any significant past medical history first presented to our facility with a traumatic skull fracture and severe eye injury. He required enucleation and subsequently underwent oculoplasty but had no other relevant surgeries or hospitalizations.
He presented to another hospital 5 years later complaining of headache, vomiting and decreased level of consciousness. Computed tomography (CT) scan revealed hydrocephalus and right thalamic hyperdense lesion with perifocal edema that was causing mass effect and some midline shift. A ventriculoperitoneal shunt (VPS) was inserted.
The patient was transferred to our institute for additional work-up. The patient was conscious on arrival to the hospital with a Glasgow coma scale of 15/15. His vital signs were stable with normal neurological examination. MRI revealed a large right thalamic lesion compressing the lateral ventricles (Fig. 1A).
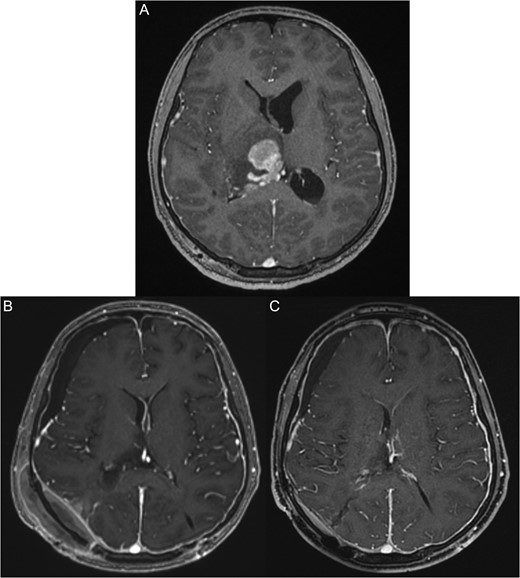
(A) Pre-operative magnetic resonant imaging of the brain showing hyperintense large right thalamic lesion compressing the lateral ventricles. (B) Three days post-operative magnetic resonant imaging of the brain showing almost total resection expect few small inhasing nodules. (C) Two months post-chemotherapy and radiotherapy, magnetic resonant imaging show total disappearance of the mass and good radiological response.
The patient underwent stereotactic biopsy for the right thalamic lesion. The histopathological examination was inconclusive because of inadequate material for immunohistochemical staining, but it indicated possible high-grade glioma versus lymphoma. These differentials were suggested based on the morphological features, including discohesive pleomorphic cells, reactive lymphocytes, necrosis and vascular proliferation.
The patient underwent cerebrospinal fluid (CSF) cytological examination because of the suspicion of the differential diagnosis, and the results revealed benign mature lymphocytes with no malignant cells. Bone marrow biopsy also revealed no evidence of lymphoma.
The patient complain of worsening headache, progressive left side weakness, vomiting and a decreased level of consciousness. CT scan revealed active hydrocephalus with enlarged ventricles and the thalamic lesion, which appeared enlarged compared to the previous scan. The VP shunt was revised, after which he developed intraventricular hemorrhage. Therefore, an external ventricular drain was inserted. His consciousness level improved post-operatively, but his weakness progressed with a high-grade fever. Septic testing was positive for CSF culture, and antibiotics were initiated. The patient completed the antibiotic course, and his condition stabilized. He underwent thalamic lesion resection via parietoccipital craniotomy using an intraventricular approach.
Frozen section examination of the lesion revealed a malignant round blue cell tumor with lymphoma as the top differential diagnosis. Histopathological examination of the surgical specimen revealed large, mitotically active, discohesive cells with abundant pale to eosinophilic cytoplasm admixed with benign reactive lymphocytes.
These atypical cells were positive for immunohistochemical staining of placental alkaline phosphatase (PLAP) and CD117 (c.kit). All morphological and immunohistochemical features supported a diagnosis of germinoma (Fig. 2). Serum alpha fetoprotein level was 1.8 μg/L (normal: 0.0–7.0), and serum beta-human chorionic gonadotropin level was 11.45 IU/L (normal: 0.00–2.00).
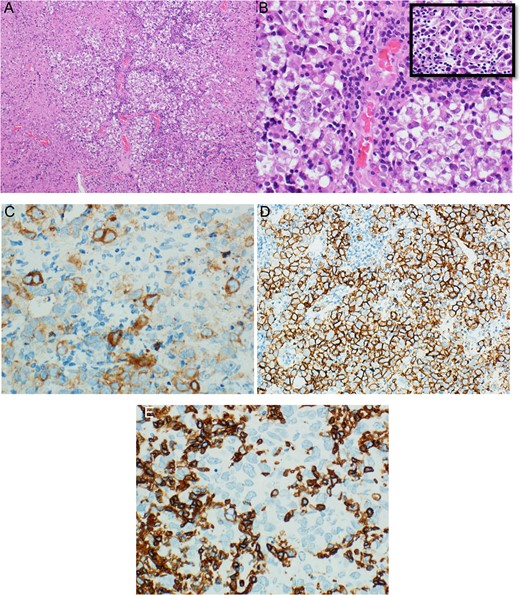
(A) Low magnification section from thalamic germinoma showed neoplastic cells arranged in large lobules separated by delicate lymphocyte-rich fibro-vascular septa. (B) High magnification of the same section showed uniform population of large polygonal cells with pale to clear cytoplasm, large centrally located vesicular nucleus, some nuclei were containing one or more prominent nucleoli, (inset) atypical mitosis neoplastic cells were positive for PLAP (C) and CD117 (D), while reactive lymphocytes were positive for CD45 (E).
The patient was transferred to the Oncology Department and received chemotherapy and radiotherapy with excellent radiological response. However, his left side weakness did not improve (Fig. 1B and C).
DISCUSSION
The thalamus and basal ganglia are rare locations for intracranial GTC. Previous reports estimated the incidence of all intracranial germinoma in the thalamus and basal ganglia between 4 and 20% [3, 5]. Thalamus and basal ganglia germinomas show a male predominance and present as a single tumor in most reported cases [2, 4].
Progressive weakness, nausea and vomiting are the most common clinical presentation. Some cases report that the disease progresses for 2 years until the time of diagnosis [4, 5]. A rapid clinical deterioration is generally associated with intratumor bleeding. Most thalamic and basal ganglia germinoma cases are not associated with endocrine disturbances [2]. These findings are consistent with our case presentation.
Some studies reported normal values of serum alpha fetoprotein and human chorionic gonadotropin. Therefore, correlations with MRI and CT findings should be performed [2, 5]. These tumors generally appear as a solid mass with variable sizes because it is a slowly growing tumor. Some studies reported mixed tumors with a cystic portion and intratumoral calcifications. The prevalence of cystic formation is ~50–60% in larger reports, which suggest that the cystic formation and calcification are generally signs of an early formation. These tumors are rarely associated with bleeding, but bleeding is associated with significant deterioration. The mass effect of the tumor on the aqueduct may cause hydrocephalus, which in some cases extends to the sella area and causes endocrinological abnormalities [3, 4]. The first presentation in our case was hydrocephalus which was treated with a VP shunt.
The diagnosis of these cases based on radiological features is generally difficult because of the wide differential, which includes malignant glioma and lymphoma [6]. These differential diagnoses were considered in our case during work-up.
Thalamic and basal ganglia germinomas are associated with ipsilateral hemisphere atrophy in 33% of cases [2]. Hemisphere atrophy from the shrinking of the internal capsule and a compensatory enlargement of sulci and ventricles are observed. Many hypotheses have been proposed, and the most common proposal is that the tumor compresses the basal ganglia and thalamic fibers, which leads to the development of Wallerian degeneration [2, 5]. One report presented a patient with hemispheric atrophy with deceased blood flow in the affected hemisphere, primarily in middle cerebral artery territories in a single-photon emission CT scan [7]. These findings present to some degree during the treatment for most of these cases [7].
The radiological diagnosis of thalamic and basal ganglia germinoma is challenging, and surgical intervention will always provide a clear answer [1, 2]. Most reports advise tumor resection to obtain intraoperative frozen biopsies, but some reports advise biopsy only to minimize surgical morbidity [2]. The amount of tissue obtained from stereotactic biopsy sometimes not sufficient [8]. The first biopsy in our case was not conclusive and delayed treatment. One report found that up to 50% of the germinoma cases exhibited other cell types, and these cells increased the resistance to radiation therapy and increased the risk of recurrence post-treatment. This report advised surgical debulking in these cases to decrease resistance and risk of recurrence [9].
Radiation therapy without a tissue diagnosis was proposed in the literature, and it is allowed only based on a good clinical diagnosis with positive tumor markers [1, 9]. Radiation alone was the proposed treatment in most reported cases as the first line of therapy with a good response. Only one report mentioned post-radiation weakness in one patient [2]. Some reports suggest chemotherapy then radiation to decrease the amount of radiation needed [2, 9].
In conclusion, diagnosis of thalamic germinoma is challenging because of the non-specific symptoms, rare location and inconclusive radiological findings. An early tissue diagnosis and a sufficient surgical biopsy are associated with early treatment and good outcome. Trial radiation may be performed in limited cases if surgical diagnosis is not feasible.
CONFLICT OF INTEREST STATEMENT
We declare that this article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.



