-
PDF
- Split View
-
Views
-
Cite
Cite
Vasileios Leivaditis, Efstatios Koletsis, Konstantinos Spiliotopoulos, Konstantinos Grapatsas, Vasiliki Tzelepi, Dimitrios Dougenis, A rare case of giant cell lung carcinoma with intracardiac extension via the pulmonary vein and thrombus formation, Journal of Surgical Case Reports, Volume 2018, Issue 6, June 2018, rjy144, https://doi.org/10.1093/jscr/rjy144
Close - Share Icon Share
Abstract
A 61-year-old man presented with dyspnea, left thoracic pain and productive cough. Chest computed tomography demonstrated a solid mass of the left upper lobe, 2.9 × 1.8 cm2 in size, which had irregular borders and appeared to infiltrate and totally occlude the upper left pulmonary vein extending up to the left atrium (LA) with thrombus formation. The patient underwent median sternotomy and left pneumonectomy, combined with LA thrombus resection under cardiopulmonary bypass (CPB) with bicaval cannulation. The LA was partially resected and the intracavitary thrombus was completely removed. The surgical margins were free of tumor cells. Episodes of embolism were not observed during surgery. The patient was successfully weaned from CPB. The postoperative course was uncomplicated. Pathological examination of the resected specimen revealed giant cell carcinoma.
INTRODUCTION
Secondary cardiac malignancies are rare but severe clinical entities. Malignant tumors may spread to the heart through direct infiltration, hematogenous metastasis, lymphatic metastasis and transvenous extension. For example renal cell, testicular and hepatocellular carcinomas may occasionally extend into the inferior vena cava and progressively grow into the right atrium. Lung tumors may directly invade the heart through the pulmonary veins. This is a rare condition, particularly when the lung tumor is small in size and its location distant from the heart [1–3]. Cardiac invasion is generally associated with a poor prognosis [4].
Tumors may also invade the pulmonary artery. Tumors growing into the pulmonary arteries include angiosarcoma and tumor embolizations from other organs [5, 6]. They represent a rare but important differential diagnosis of pulmonary thromboembolism [5–7].
Herein we report a case of an intracardiac extension of a tumor via the pulmonary veins. Several such cases have been reported in the existing literature [4, 5, 8–10].
CASE REPORT
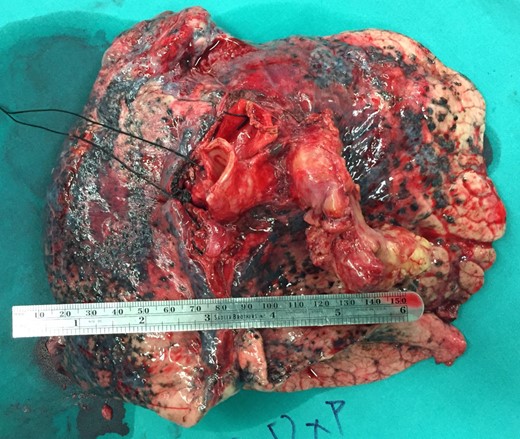
A 61-year-old male, with a history of heavy tobacco abuse presented with dyspnea, left thoracic pain and productive cough. He did not report palpitations or hemoptysis. Chest radiography showed an abnormal shadow in the left upper lung field. Computed tomography (CT) scan revealed a solid lesion of the left upper lobe, 2.9 × 1.8 cm2 in size, with irregular borders (Fig. 1a and c). The tumor seemed to infiltrate the upper left pulmonary vein which appeared dilated and totally occluded due to the presence of thrombotic material in its lumen (Fig. 1b and d). Transthoracic echocardiography showed an intracardiac structure, 4 × 2.5 cm2 in size, protruding from the left pulmonary vein, having no adhesions to the atrial walls. Magnetic resonance imaging (MRI) confirmed the above findings. (Fig 2a–d). A positron emission tomography scan (PET) was also performed. It confirmed the CT findings showing an irregular nodular lesion in the left upper lobe with irregular margins and a diameter of 2.8 cm. It showed a hypermetabolic activity (SUVmax = 29.6) (Fig. 3a and c). It also showed a pleural lesion ventrally to the upper lobe parenchyma. Another hypermetabolic focus (SUVmax = 21.4) appeared in the lumen of the upper lobe pulmonary vein (Fig. 3b and c). Bronchoscopy showed the presence of abnormal tissue in the apicoposterior segmental bronchus of the left upper lobe. Tissue biopsies were obtained but showed no signs of malignancy. Spirometry revealed a mild obstructive pattern. A complete staging work-up, including head CT scan, abdominal ultrasound and whole body bone scintigraphy, did not show any distant metastasis. Based on these the preoperative staging was T4N0M0. Despite failure to obtain a pathologic diagnosis, a surgical treatment including left pneumonectomy with entry in the left atrium and removal of the intracardiac thrombus under cardiopulmonary bypass (CPB) was decided based on the potential immediate life-threatening situation. The presence of such an intracardiac thrombus could be associated with secondary migration, acute blockage of the mitral valve and heart failure, or a distal arterial embolisation resulting into a fatal stroke, a mesenteric ischemia or a severe peripheral ischemia [2, 4]. Median sternotomy was the surgical approach and the patient was cannulated using bicaval venous return and underwent CPB. Left pneumonectomy was carried out, initially with left pulmonary artery ligation and resection, followed by resection of the left pulmonary veins and part of the wall of the left atrium together with the intraatrial mass, which was removed en bloc. The left atrium was sutured closed and then the left main bronchus was resected, completing the left pneumonectomy. The nodal stations 4, 5, 7, 8 and 9 were also sampled. The surgical specimen (Fig. 4) showed the same features as those seen on the CT scan. The patient was successfully extubated directly after the operation and was transferred to the ward on the first postoperative day. The patient was discharged on the 12th postoperative day and received adjuvant chemotherapy thereafter.
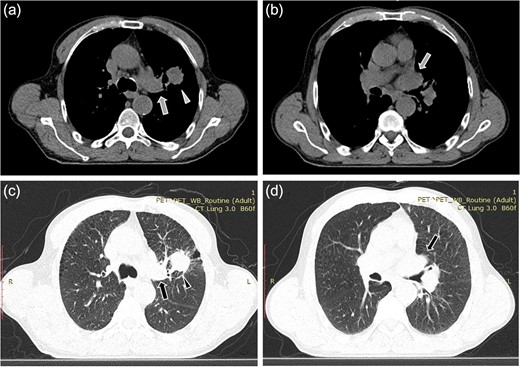
Computed tomopgraphy images showing the solid lesion (triangular pointer) with irregular borders in the left upper lobe (a, c). The tumor infiltrates the upper left pulmonary vein (arrow) which appears dilated and totally occluded due to the presence of the thrombus in its lumen (b, d).
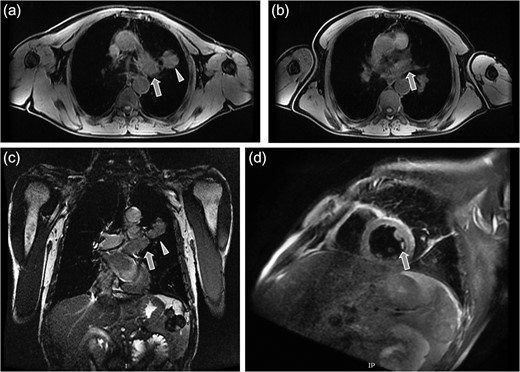
Magnetic resonance tomography images which confirmed the CT findings. (a) Axial plane showing the irregular solid mass in the left upper lobe (triangular pointer). (b and c) Axial and coronal plane showing the dilation and occlusion of the left upper pulmonary vein (arrow). (d) Sagital reconstruction revealing the dilation of the left upper pulmonary vein and the presence of thrombotic material in its lumen (arrow).
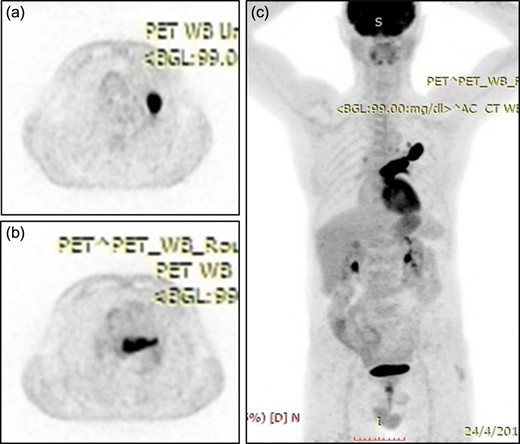
(a) Positron emission tomography scan showing an irregular nodular lesion in the left upper lobe with irregular margins and hypermetabolic activity. (b) Hypermetabolic focus in the lumen of the upper lobe pulmonary vein. (c) Coronar reconstruction showing the focal hypermetabolic activity in the left upper lobe, the left atrium and the left upper pulmonary vein.
Pathological examination of the surgical specimen showed that the tumor was composed of a diffuse arrangement of discohesive pleomorphic cells with large nuclei, prominent nucleoli and eosinophilic cytoplasm with emperipolesis (Fig. 5). Some tumor cells had lobulated nuclei whereas others were multinucleated (giant cells). A diagnosis of a giant cell carcinoma was rendered. Focally, in one of the sections, solid nodules of carcinoma cells that were less pleomorphic and had smaller size than the cells described above were noted (Fig. 5). Immunohistochemistry was performed. Clone and sources of the antibodies used are shown in Table 1. The envision detection system was used (Dako, Denmark). The giant cell carcinoma cells were negative for the cytokeratin cocktails AE1 and AE3, as well as for cytokeratins CK7 (Fig. 6) and CK5/6. They were also negative for p63, TTF1 (Fig. 6), napsin and LCA. The smaller sized component expressed diffusively AE1, AE3, CK7, TTF1 (Fig. 6) and napsin. It was also faintly positive for p63 and CK5/6. A diagnosis of a giant cell carcinoma of the lung with focal adenocarcinoma component was rendered. Lymph node from lymph node station 5 was focally infiltrated by the tumor. All other lymph nodes examined were negative for malignancy. Surgical margins were not involved by tumor. Pathologic stage was pT4N2.
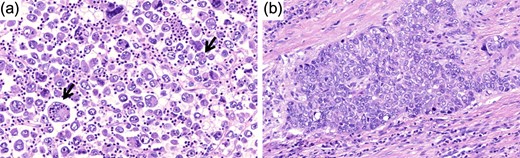
Hematoxylin and eosin image of the tumor (original magnification ×25). (a) Note the emperipolesis in the giant cell component (arrow). (b) The more differentiated component that consisted <5% of the tumor is shown here.
| Antibody . | Clone . | Source . |
|---|---|---|
| Cytokeratin, LMW | AE1 | Cell Marque, USA |
| Cytokeratin, HMW | AE3 | Cell Marque, USA |
| CK7 | PA0942 | Leica, USA |
| CK5/6 | CK5/6.007 | Biocare, USA |
| TTF1 | 8G7G3/1 | Dako, Denmark |
| napsin | ERP 6252 | Thermo Scientific, USA |
| p63 | DAK-p63 | Dako, Denmark |
| LCA | LCA88 | Biogenex, USA |
| Antibody . | Clone . | Source . |
|---|---|---|
| Cytokeratin, LMW | AE1 | Cell Marque, USA |
| Cytokeratin, HMW | AE3 | Cell Marque, USA |
| CK7 | PA0942 | Leica, USA |
| CK5/6 | CK5/6.007 | Biocare, USA |
| TTF1 | 8G7G3/1 | Dako, Denmark |
| napsin | ERP 6252 | Thermo Scientific, USA |
| p63 | DAK-p63 | Dako, Denmark |
| LCA | LCA88 | Biogenex, USA |
| Antibody . | Clone . | Source . |
|---|---|---|
| Cytokeratin, LMW | AE1 | Cell Marque, USA |
| Cytokeratin, HMW | AE3 | Cell Marque, USA |
| CK7 | PA0942 | Leica, USA |
| CK5/6 | CK5/6.007 | Biocare, USA |
| TTF1 | 8G7G3/1 | Dako, Denmark |
| napsin | ERP 6252 | Thermo Scientific, USA |
| p63 | DAK-p63 | Dako, Denmark |
| LCA | LCA88 | Biogenex, USA |
| Antibody . | Clone . | Source . |
|---|---|---|
| Cytokeratin, LMW | AE1 | Cell Marque, USA |
| Cytokeratin, HMW | AE3 | Cell Marque, USA |
| CK7 | PA0942 | Leica, USA |
| CK5/6 | CK5/6.007 | Biocare, USA |
| TTF1 | 8G7G3/1 | Dako, Denmark |
| napsin | ERP 6252 | Thermo Scientific, USA |
| p63 | DAK-p63 | Dako, Denmark |
| LCA | LCA88 | Biogenex, USA |
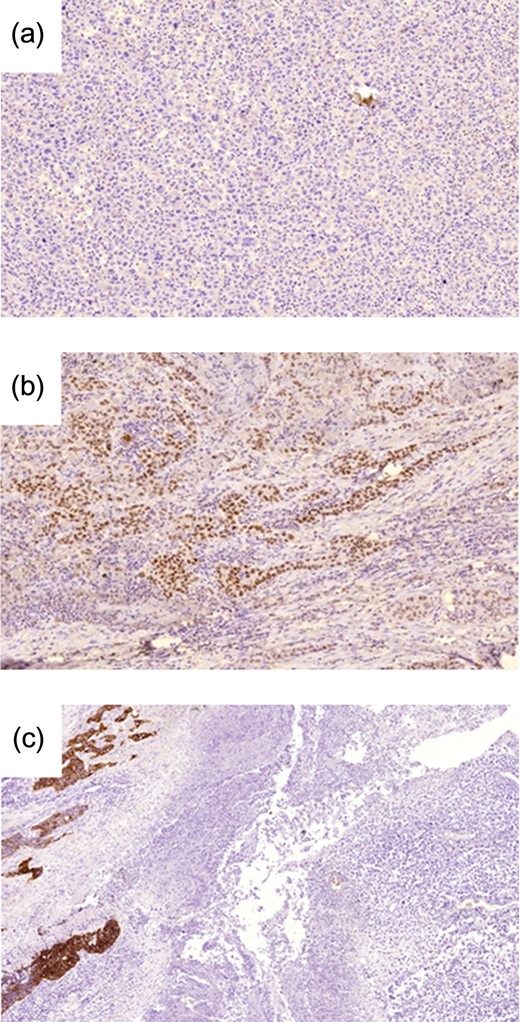
(a) TTF1 expression is negative in the giant cell component (original magnification ×10). (b) Positive expression in the adenocarcinoma component (original magnification ×10). (c) CK7 expression is noted in the adenocarcinoma component (left) but is negative in the giant cell component (right) (original magnification ×5).
DISCUSSION
Intravenous extension of the tumor into the heart is a rare complication of lung cancers associated with poor prognosis and several cases including small cell carcinoma and non-small cell carcinomas have been reported in the current literature. Some of them presented with circulatory and respiratory symptoms [5, 8–10].
Several reports in the literature mention the occurrence of intracardiac neoplastic thrombi but the vast majority of these thrombi are located in the right side of the heart. They are classically reported in association with renal cell, testicular or hepatocellular carcinoma, related to tumor emboli or endovascular progression of the tumor [1–3]. Thrombus formation in the pulmonary artery due to direct invasion of the tumor in the artery has also been reported [6]. An anticoagulation therapy is usually the therapy of choice in cases of intracardiac thrombi. In our case, the occurrence of a malignant thrombus in the left upper pulmonary vein with an intracardiac extension seems to be caused by a direct intravascular spread of the disease. Such patients are in high risk of sudden cardiac arrest due to inflow obstruction in the mitral valve, or massive tumor emboli involving major organs [4, 9]. A surgical treatment was therefore indicated.
We decided to carry out the operation under CPB, due to the intracardiac extension of the tumor thrombus, hence ruling out vascular clamping and avoiding the risk of thrombus dislocation, and embolism. CPB can, however, also be combined with vascular clamping in some cases. Under CPB the tension of the LA is significantly removed. Bradycardia which can be induced by hypothermia could also reduce the risk of clamp dislocation. Moreover, bleeding complications or injury of the LA wall can be dealt with more effectively and appropriately with the use of CPB [8].
CONCLUSIONS
In conclusion, this is a rare case of a giant cell lung cancer. This mode of cancer extension is rare and its prognostic significance remains unclear. Although pulmonary vein invasion is generally considered as a negative prognostic sign, it demands surgical exploration for diagnosis and a definitive treatment.
CONFLICT OF INTEREST STATEMENT
None declared.
DISCLOSURES
The authors of this article have no relevant financial or non-financial relationships to disclose.



