-
PDF
- Split View
-
Views
-
Cite
Cite
Hussein Nassar, Ahmad Zaghal, Ali Taher, Rami Mahfouz, Bassem Safadi, Mariam Kanso, Mohammad Khalife, Walid Faraj, Triple thrombophilic simultaneous mutations in patients after bariatric surgery: is there a role for screening in the Eastern Mediterranean?, Journal of Surgical Case Reports, Volume 2018, Issue 6, June 2018, rjy135, https://doi.org/10.1093/jscr/rjy135
Close - Share Icon Share
Abstract
Background and purpose: Thrombophilia is a hypercoagulable state that predisposes to thrombosis. Several genetic risk factors have been shown to predispose to thromboembolic events. Homozygosity to a thrombophilic mutation certainly predisposes the affected patient to more serious symptoms. Materials and methods: Here we present a case of a 56-year-old male patient who underwent sleeve gastrectomy for morbid obesity, presenting to our institution with abdominal pain. Investigations revealed thrombosis of the splenic, axillary vein as well as the right pulmonary artery. The patient was found to have triple thrombophilic mutations. Results: To our knowledge, this is the first reported case of three specific simultaneous thrombophilic mutations in a patient from the Eastern Middle East. Conclusion: We suggest a role for screening for thrombophilic mutations in the Eastern Mediterranean patients undergoing bariatric surgeries for morbid obesity due to the increased risk of thrombosis in this group of patients
INTRODUCTION
Thrombophilia is a hypercoagulable state that predisposes to thrombosis [1]. In addition to the well-established acquired risk factors for venous thromboembolic events, several genetic risk factors such as factor V Leiden (FV Leiden) (G1691A), prothrombin gene (factor II) (PTH) (G20210A) and MethyleneTetrahydrofolate Reductase (MTHFR) (C677T) mutations have been shown to predispose to thromboembolic events [1]. Patients with one or more of these mutations are at risk of developing venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), or developing adverse pregnancy outcomes including recurrent abortions, severe pre-eclampsia, intrauterine growth restriction (IUGR) and premature delivery [2]. Bariatric surgery patients are at elevated risk for thromboembolic events because of underlying venous stasis, immobility, increased intra-abdominal pressure and the risk of surgery itself [2]. Studies have shown that PE accounts for as many as half of all deaths after Roun-en-Y Gastric bypass surgery [3].
We report a case of a 56-year-old male patient presenting with simultaneous multiple thromboembolic events and was found to have three major thrombophilia gene co-mutations in FV Leiden, PTH and methylenetetrahydrofolate reductase genes.
CASE PRESENTATION
A 56-year-old male patient underwent sleeve gastrectomy one year prior to presentation, presented to our institution with left upper abdominal pain of few days duration. There was no history of chest pain or dyspnea, there were no focal neurological symptoms. On physical examination, the patient had left upper quadrant abdominal tenderness, no rebound tenderness or guarding. The patient had no known previous history of thromboembolic events.
Laboratory values on presentation were within normal limits, coagulation profile: INR = 1.4, aPTT = 35.5 s, PT = 16.6 s, Anithrombin III = 96%, protein S = 104%, potein C = 65%.
A computed tomography scan of the chest, abdomen and pelvis revealed splenic vein thrombosis (Fig. 1); and a filling defect in the lower lobar segmental branch of the right pulmonary artery consistent with acute segmental pulmonary embolism. A venous duplex scan of the extremities showed thrombosis of the left axillary and basilic veins (Figs 2 and 3). Echocardiogram was normal.
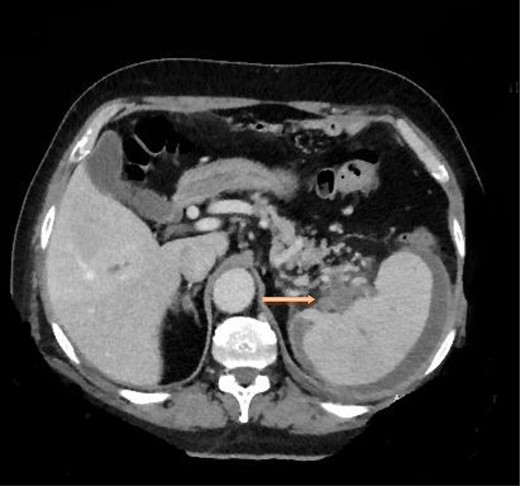
Computed tomography scan of the abdomen with evidence of splenic vein thrombosis at the splenic hilum.
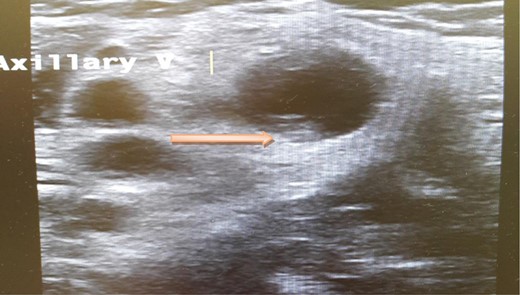
Ultrasound scan of the axillary vein with evidence of thrombus formation.
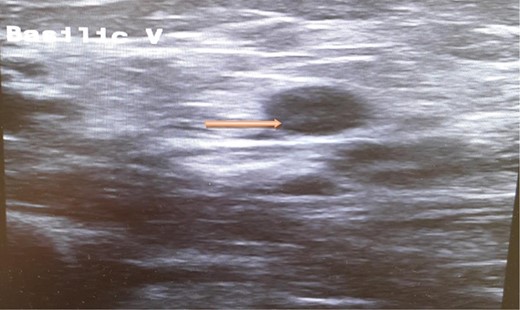
Ultrasound scan of the basilic vein with evidence of thrombus formation.
The presence of multiple simultaneous thrombotic events in this patient triggered molecular work-up: heterozygous for Factor V Leiden (G1691A) mutation, Factor II (G20210A) mutation and MTHFR (C677T) mutation. The patient was started on therapeutic dose of subcutaneous low molecular weight heparin (LMWH), hospitalized for 4 days, discharged home on LMWH bridged to oral acenocoumarol, on which he was maintained for 6 months.
DISCUSSION
Genetic profiling for patients with thromboembolic events is frequently performed [2]. Factor V Leiden is the most common predisposing factor, accounting for 40–50% of the cases. The prothrombin (Factor II) gene mutation, deficiencies in protein S, protein C and antithrombin account for most of the remaining cases. In Lebanon, the prevalence of FV Leiden is 14% among healthy individuals and 40% among patients with DVT [4].
Double heterozygosity for FV Leiden and factor II mutation (G20210A) is the most common genetic combination with an increased risk of thrombosis [2]. This increased risk of thrombosis could be explained by the synergistic effect between the two mutations. Triple mutations involving these three genes remains a rare entity, as is the case with our patient herein reported. A study conducted on Omani population showed no FV Leiden and prothrombin gene mutations in any subject. However, MTHFR and CBS 844ins68 along with protein C deficiency were important risk factors. More importantly, 56.4% of these cases had either one or a combination of these three mutations [5]. Multiple studies have evaluated the role of FV Leiden in the risk of VTE. Approximately 10–26% of patients with VTE are carriers of the factor V Leiden mutation [5]. Heterozygosity for FV Leiden mutation increases the risk of venous thrombosis 5–10 times, while with homozygosity for the mutation there is an 80–100 times increased risk for venous thrombosis [6]. Prothrombin gene mutation G20210A causes elevated levels of Prothrombin (factor II) and thus increased risk for venous thrombosis [7]. Patients with MTHFR gene mutation may develop more than one thrombophilic gene mutation resulting in a higher risk of thrombosis than those with a single gene mutation [8]. In addition, carriers of the G20210A prothrombin gene mutation have an increased risk of deep vein, hepatic, portal, mesenteric, cerebral vein thrombosis and an increased risk to pulmonary emboli [9].
Bariatric surgery candidates have high rates of acquired as well as inherited thrombophilias [3]. Data are still not clear on whether to proceed with preoperative VTE screening through laboratory studies and imaging, in the setting of improved perioperative prophylaxis for thromboembolism in all bariatric surgery patients [3]. Data are still not clear on whether to proceed with preoperative VTE screening through laboratory studies and imaging, in the setting of improved perioperative prophylaxis for thromboembolism in all bariatric surgery patients. For instance, several studies have employed profiling of VTE risk through laboratory testing for thrombophilias in all patients, through studying D-Dimer, Fibrinogen, Factor VIII, Factor IX and Factor XI, and other factors, in addition to preoperative imaging with Dupplex [2, 9].
Anticoagulation options for acute thromboembolic events include unfractionated heparin, LMWH, fondaparinux and the direct oral anticoagulants (DOACs) with equivalent efficacy [10].
To note, the assay done at our institution detects only the FV Leiden G1691A mutation, Factor II (Prothrombin) G20210A mutation and the MTHFR C677T mutation. Heterozygosity for MTHFR by itself has not been found to contribute to thrombosis, however, it is important to note that other mutations may be associated to this gene and may lead to an increased homocysteine level. In the case of our patient, homocysteine level was mildly elevated (15 μmol/L). A variety of other genetic and non-genetic factors may contribute to increased risk of thrombosis.
CONCLUSION
Here we report a rare case of triple thrombophilic mutations associated with multiple thrombophilic episodes. We suggest a role for screening for thrombophilic mutations in the Eastern Mediterranean patients undergoing bariatric surgeries for morbid obesity due to the increased risk of thrombosis in this group of patients.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL STATEMENT
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT STATEMENT
Informed consent was obtained from all individual participants (one patient) included in the study.



