-
PDF
- Split View
-
Views
-
Cite
Cite
Preci L Hamilton, Garth Cruickshank, Delayed central nervous system manifestation of Chikungunya virus with magnetic resonance T2 weighted imaging high signal changes—a case report, Journal of Surgical Case Reports, Volume 2018, Issue 6, June 2018, rjy134, https://doi.org/10.1093/jscr/rjy134
Close - Share Icon Share
Abstract
CHIKV is a relatively new virus and we are still learning about the illness. Very little is known about CNS its involvement and even less about its delayed or long-term manifestations if any. It therefore behoves us to consider delayed CNS involvement when assessing patients with CHIKV infections that may not have had an acute neurological manifestation at the time of diagnosis coupled with new onset neurological manifestations and MRI abnormalities. It seems likely that patients with CHIKV may experience delayed CNS manifestation of the viral infection. This report highlights the importance of a travel history when assessing patients with a neurological complaint. The pathway to best manage such cases is with repeated imaging to assess if the signal changes either progress, resolve or more importantly if there is any MRI correlation should changes in neurology develop during the surveillance period.
INTRODUCTION
Viral infections affecting the central nervous system (CNS) have been a prominent feature in the literature since the Zika Virus (ZIKV) epidemic. Whilst much has been published about ZIKV, very little has been mentioned about the CNS impact of Chikungunya virus (CHIKV). CHIKV is an RNA virus from the Togaviridae family [1]. Its vector is the female Aedes egypti mosquito and usually manifests as a self-limiting disease associated with uveitis, retinitis, myalgia and polyarthralgia [2]. Early reports of CHIKV date back to Africa and India in the 1950s and 1960s, respectively, but the first confirmed case in Caribbean was in December 2013 [3, 4]. Unlike the Zika virus, no neurological involvement of CHIKV was reported in Jamaica despite the 2014 epidemic affecting ~60% of the population.
With the shrinking world phenomenon of globalization, Jamaicans and other Caribbean nationals increasingly venture to the global north for studies, business or simply to visit whilst tourists are attracted to the caribbean for its beaches and tropical weather [4–6]. Despite this bilateral travel exchange, there has been no report addressing neither CHIKV in the Jamaican diaspora nor its long-term delayed CNS manifestations. To the best of our knowledge this is the first report addressing both of these issues.
CASE REPORT
We report the case of a 77-year-old woman of West Indian origin who was clinically diagnosed with Chikungunya having experienced a period of malaise during the 2014 CHIKV epidemic in Jamaica. She had no neurological involvement and was better after 1 week of taking Paracetamol. After returning to the UK, she continued to experience occasional headaches, polyarthralgia and fatigue and went on to developed first episode of generalized tonic–clonic seizures in June 2015 requiring admission to the Intensive Treatment Unit where seizures were aborted using Phenytoin. In hospital, she had unremarkable lab investigations including lumbar puncture done, N-methyl d-Aspartate receptor (NMDA) and voltage gated potassium channel (VGKC) antibody titres. She regained consciousness after 5 days and since then suffered multiple episodes of seizures with inter-ictal confusion and was treated with Levetiracetam, which was discontinued after being seizure-free for several weeks.
November 2015, she had another seizure episode and MRI brain at that time was suspicious for a right mesial temporal lobe low-grade glioma (Fig. 1). She had no pre-seizure personality changes, hearing difficulty or family history of either seizure disorder or brain or any other tumour. Her neurological screening examination was unremarkable.
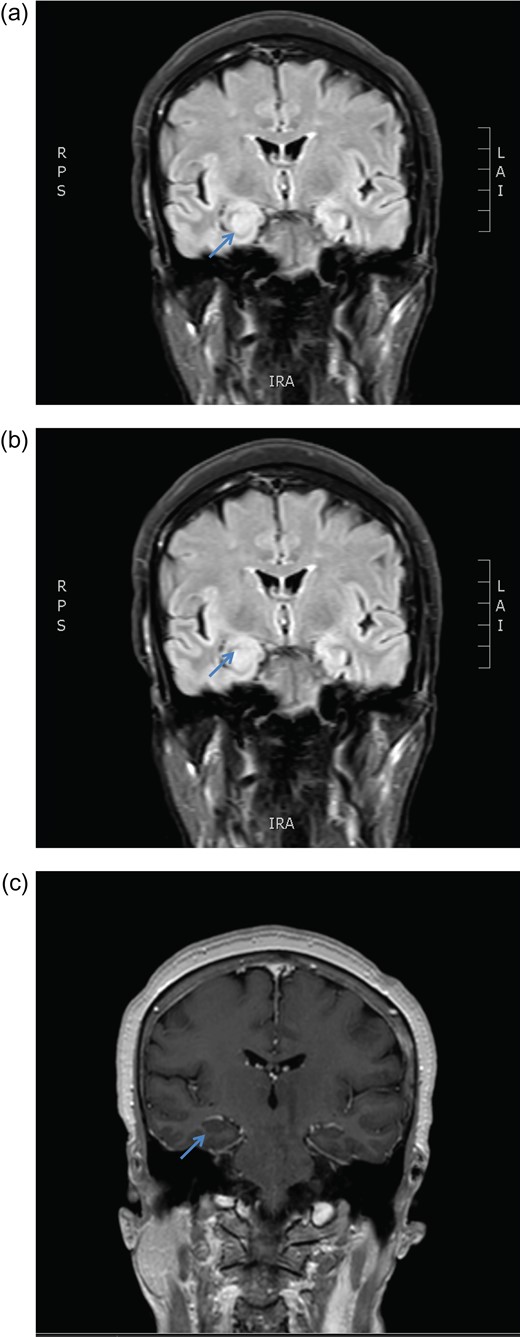
MRI brain coronal T2 FLAIR sequence depicting persistent right hippocampal high signal change in January (a) and March (b) 2016. There is no enhancement post gadolinium injection (c).
December 2015, she had a normal electroencephalogram (EEG). Subsequent MRI done January and March 2016 showed T2 Weighted Imaging Fluid Attenuated Inversion Recovery (T2WI/FLAIR) changes in the right medial temporal lobe (Fig. 2). Right hippocampal signal change was suspicious of low-grade glioma with a differential diagnosis of post viral limbic encephalitis. There was no enhancement with intravenous gadolinium administration and no other suggestion of a focal malignant disease. She remained clinically well and repeat MRI done October 2016 showed areas of signal abnormalities focused around the posterior horns of the lateral ventricles and but no sign of right temporal lobe low-grade glioma or encephalitis (Fig. 3). All of these signal changes resolved completely with repeated MRI and she had no further seizures.
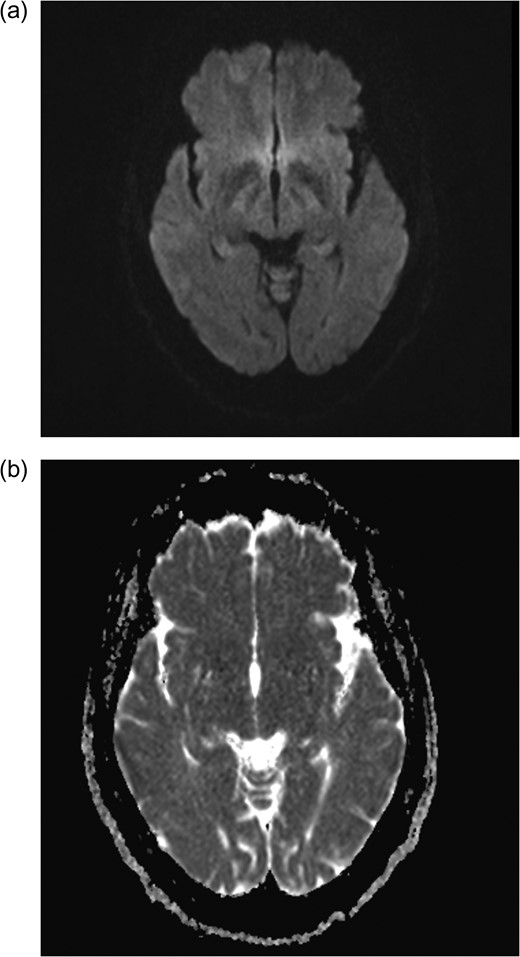
Axial MRI brain (a) diffusion weighted imaging (DWI) and (b) apparent diffusion coefficient (ADC) demonstrating no restricted diffusion.
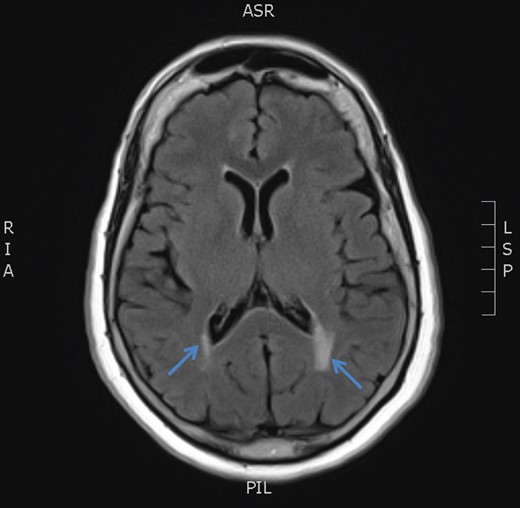
MRI brain axial T2 FLAIR sequence (October 2016) depicting resolution of right medial temporal lobe high signal but prominence of periventricular hyperintensity in periventricular white matter (arrows).
DISCUSSION
Her referral to our neurosurgical team was for evaluation of a low-grade glioma, based on her abnormal non enhancing T1 and T2 changes. Although seizures are a frequent presenting feature of gliomas, it would be unusual but not impossible for a 77-year-old to present with low-grade glioma. It is far more common for the gliomas in older patients to present as high-grade lesions. The periventricular appearance might also suggest a diagnosis of lymphoma however; the absence of contrast enhancement would seem to rule out both of these neoplasms.
Since Mazaud reported the acute neurological effect of CHIKV in 1971 only a few other scholars mentioned the topic [7]. Reported delayed CNS effects include Guillian Barre Syndrome, and myelitis but these delayed presentations were by weeks and not months or years as in this patient’s case [8]. It is established that CHIKV can cause acute neurological involvement but there are no reported case of CHIKV having its CNS manifestation delayed by months or years. This may be the case in this patient who experienced seizures 18 months after being diagnosed with CHIKV. It is, however, a relatively new virus and we are still learning about its systemic effects.
It is reasonable to consider limbic encephalitis given that she has mesial temporal lobe T2WI abnormality coupled with seizures having had a viral illness in the past. However, this is less likely given that NMDA and VGKC antibody titres were negative. No history of a tumour would also suggest that anti-NMDA encephalitis less likely but remained arguable as 80% of these patients had no demonstrable tumour [9].
It was reasonable to entertain an association with CHIKV as one sought to elucidate the cause of her seizures after 7 decades. Her case differed from those reported by Ganesan [10] in that she had seizures more than one year following diagnosis of CHIKV, normal EEG and CSF analysis, and she did not deteriorated unlike his first case. Additionally, he reported bilateral contrast enhancing frontal T2WI changes with restricted diffusion. She had resolution of the initial abnormality seen on T2WI/FLAIR sequence without steroid administration.
Very little is known about its CNS involvement and even less about its delayed and long-term manifestations if any. It seemed likely that this woman’s presentation was related to her episode of CHIKV and may have represented a delayed CNS manifestation. The pathway to best manage her was with repeated imaging rather than a biopsy. This case underscores the paramount importance of a travel history particularly to endemic territories in patients with neurological manifestations.
Conflict of Interest statement
None declared.



