-
PDF
- Split View
-
Views
-
Cite
Cite
Contardo Vergani, Maria Elisa Messina, Irene Giusti, Marco Venturi, An incredibly dilated Wirsung mimicking a sero-cystic neoplasm of the pancreatic head, Journal of Surgical Case Reports, Volume 2018, Issue 6, June 2018, rjy122, https://doi.org/10.1093/jscr/rjy122
Close - Share Icon Share
Abstract
A diabetic patient who at a routine abdominal ultrasounds was found to have a very dilated pancreatic duct. Computed tomography (CT) scan diagnosed a sero-cystic lesion of the pancreatic head. Gastroduodenoscopy discovered a duodenal hyperemic area, which was sampled. Biopsy demonstrated intramucosal vascular emboli from a neuroendocrine carcinoma positive for Chromogranin A and Somatostatin and negative for Gastrin. Cholangio-magnetic resonance imaging revealed that the sero-cystic lesion found at CT, was being mimicked by the enormously dilated pancreatic duct but suggested the possibility of an intraductal or ampullar neoplasm. Blood and urine tests were not helpful and an octreoscan was negative. The patient underwent surgery. Direct exploration confirmed the severe pancreatic duct dilation and a cephalic lesion requiring a pancreatoduodenectomy. Histology confirmed a neuroendocrine tumor infiltrating the duodenum. We conclude that despite modern sophisticated imaging and endoscopic techniques, the evaluation of bilio-pancreatic region can be challenging and can reserve surgical surprises.
INTRODUCTION
Modern imaging techniques greatly facilitate the evaluation of bilio-pancreatic structures, however precise definition of pancreatic lesions can sometimes be challenging despite extensive preoperative evaluation. We describe a case of misleading imaging features followed by a surgical ‘surprise’ which prompted a different surgical strategy.
CASE REPORT
A 55-year-old asymptomatic man, with type 2 diabetes mellitus controlled by oral metformin, underwent an abdominal ultrasound that incidentally discovered a remarkable pancreatic duct dilation. The pancreatic head could not be well visualized.
The patient therefore underwent a computed tomography (CT) scan (Figs 1 and 2) that revealed ‘a thickened duodenal wall and a sero-cystic lesion of the pancreatic head without contrast-enhancement, which compressed the duodenal lumen and caused a dilation of the Wirsung duct’. The liver, the gallbladder and the extrahepatic biliary tree were normal. No lymphadenopathy was found.
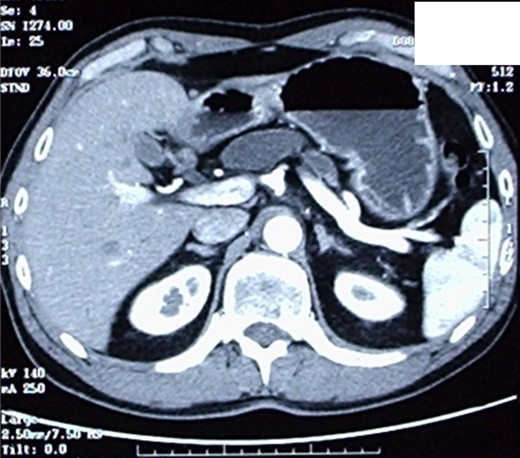
CT-scan, showing a sero-cystic lesion of the pancreatic head without contrast-enhancement, compressing the duodenal lumen and causing a dilation of the Wirsung duct.
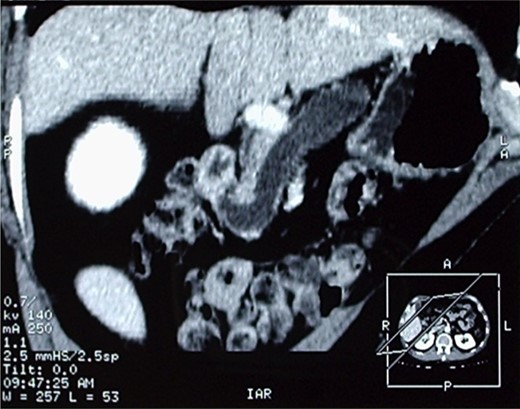
Another image of the CT scan, showing a sero-cystic lesion of the pancreatic head.
An oesophagogastroduodenoscopy showed a normal duodenal bulb and descending portion of the duodenum, but at the superior duodenal knee, a 13-mm hyperemic area was found and sampled. The endoscopist decided at that time not to perform an ERCP, judging as relevant the risk of bleeding. Histology revealed vascular emboli from a neuroendocrine carcinoma within the duodenal mucosa. Immunocytochemical tests were positive for Chromogranin A and Somatostatin; and negative for Gastrin.
Serum levels of CEA, CA 19-9, AFP and plasma levels of VIP, somatostatin, gastrin and chromogranin A were normal as were urinary levels of 5HIAA.
A Cholangio-magnetic resonance imaging (MRI) (Fig. 3) revealed that the sero-cystic lesion found at CT, was being mimicked by the enormously dilated pancreatic duct that had reached a diameter of 2 cm at the level of the pancreatic body. The dilation decreased to 1 cm in the pancreatic tail and the lumen of the Wirsung duct seemed to have an irregular profile. On the opposite side, the Wirsung caliber suddenly decreased in proximity to its duodenal end. This finding was not univocal and could suggest an intraductal mucous secreting tumor, chronic pancreatitis or an ampullary alteration. The pancreatic parenchyma of the body and of the tail was remarkably hypotrophic, and only some residual pancreatic tissue was observed at the uncinate process. The choledochus maintained a regular size almost down to its duodenal end, but in proximity of the Vater ampulla it disappeared for about one cm, suggesting a functional spasm or, more likely, a pathologic process of the ampulla. An octreoscan did not demonstrate any somatostatin receptor.
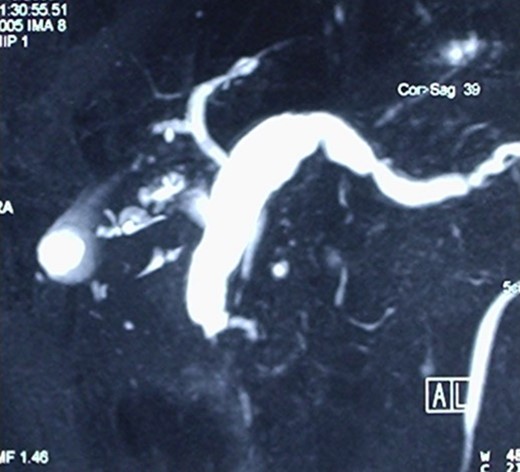
Cholangio-MRI, showing an enormously dilated pancreatic duct, with the diameter of 2 cm at the level of the pancreatic body mimicking the sero-cystic lesion found at CT.
Preoperative work-up was consistent with a neuroendocrine pancreatic tumor, probably a somatostatinoma [1, 2].
Considering the clinical picture suggesting an ampullary neoplasm around 1 cm in size, surgical treatment was planned for an intended enucleation of the presumed ampullary neoplasm [3, 4], even if the patient was informed of the possibility that a major pancreatic resection could be necessary and relevant consensus was obtained.
At duodenotomy, we actually found a 2 × 3 cm lesion of the pancreatic head, infiltrating the medial wall of the duodenum cephalad to the papilla of Vater, that was not be amenable to a limited resection. We therefore diverted to a duodenocefalopancreasectomy with pyloric preservation according to Traverso–Longmire.
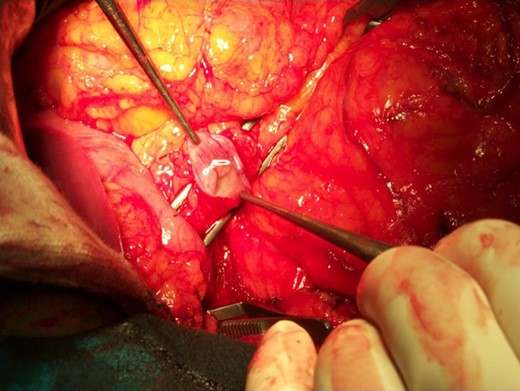
The operative specimen showed a normal thin choledocus (incannulated with a probe) cephalad to a fragile inhomogeneous area corresponding to the tumor (Fig. 4) and an enormously dilated pancreatic duct (held by the forceps) (Fig. 5).
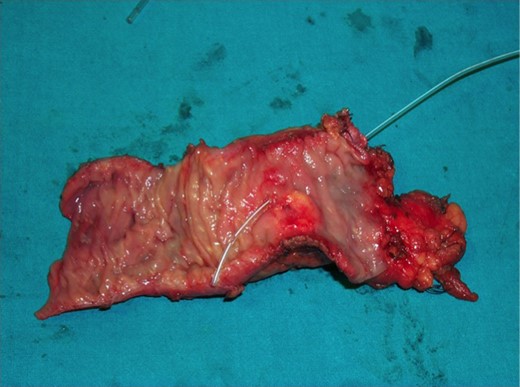
The operative specimen showing a normal thin choledocus (incannulated with a probe) cephalad to the tumor.
Post-operative histology confirmed a neuroendocrine neoplasm of the ampullary region, infiltrating the duodenal wall as well as the periduodenal and peripancreatic soft tissues. The pancreatic duct was dilated to a diameter of 2.5 cm. The pancreatic tissue was atrophic and there was diffuse sclerosis of the main pancreatic duct. Six out of 17 peripancreatic and periduodenal lymph nodes were found to have neuroendocrine metastases. The proliferating activity (MIB-1) was <2% with a number of mithosis/10HPF below 2. Immunohistochemical staining was positive for synaptophysin and somatostatin and was negative for insulin, glucagon and pancreatic polypeptide.
Post-operative course was uneventful and the patient was discharged on the 11th post-operative day.
DISCUSSION AND CONCLUSIONS
Even with the more advanced and sophisticated diagnostic imaging, endoscopic, immunological and nuclear techniques, evaluation of the ampullary region of the pancreas can be difficult and strict relationships among remarkably altered adjacent anatomic structures can be misleading [5, 6]. In this case, the lesion was interpreted by CT as a sero-cystic neoplasm, then recognized by MRI as a large dilation of the Wirsung and finally, only at duodenotomy, the cause of the Wirsung dilation could be defined. The treatment plan was consequently diverted to a duodenopancreasectomy.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- diabetes mellitus
- computed tomography
- biopsy
- endoscopy
- carcinoma, neuroendocrine
- cysts
- dilatation, pathologic
- neuroendocrine tumors
- pancreatic ducts
- pancreaticoduodenectomy
- surgical procedures, operative
- urinalysis
- diagnostic imaging
- duodenum
- histology
- neoplasms
- pancreas
- somatostatin
- vibration
- embolism
- chromogranin a
- abdominal ultrasonography
- indium in 111 pentetreotide
- pancreas head
- cephalic



