-
PDF
- Split View
-
Views
-
Cite
Cite
Neil Oxborrow, Rajkumar Sundarapandian, Heterotopic ossification following use of i-Factor for spinal fusion in Mucopolysaccharidosis 1: a case report, Journal of Surgical Case Reports, Volume 2018, Issue 6, June 2018, rjy120, https://doi.org/10.1093/jscr/rjy120
Close - Share Icon Share
Abstract
Mucopolysaccharidosis is a rare group of genetic disorder which results in a complex of anomalies involving various systems. In Mucopolysaccharidosis 1 progressive thoracolumbar kyphosis is a common presentation which can result in instability and neurological deficit. Posterior spinal surgery is performed to correct deformity and obtain spinal fusion. Peptide enhanced bone graft substitute (i-FactorTM) is relatively a new component with proven efficacy to obtain early spinal fusion. An 8-year-old child with progressive high lumbar kyphosis due to Mucopolysaccharidosis 1 was admitted for Posterior spinal fusion with i-Factor bone graft substitute. Postoperatively patient had serous discharge from the wound which settled without intervention. A month after the surgery spinal radiographs revealed heterotopic ossification at the distal end of spinal construct in the paraspinal region. Patient remained asymptomatic and clinically well.
INTRODUCTION
I-FactorTM peptide enhanced bone graft substitute has been used to stimulate early postoperative fusion in dental surgery [1] and found to be an effective alternative to autograft in orthopaedics for treating ununited long bone fractures [2]. Little is recorded with regards to its potential complications in Paediatric Spinal Surgery. We present the case report of a patient with Mucopolysaccharidosis 1, who underwent Posterior spinal fusion using i-Factor and developed heterotopic ossification.
I-Factor is a composite of bioactive P-15 peptide and anorganic bone matrix. The P-15 peptide is responsible for attachment and proliferation of osteogenic cells in fusion sites. Osteogenic cells bind to i-Factor the same way they bind to type-1 collagen [3].
CASE REPORT
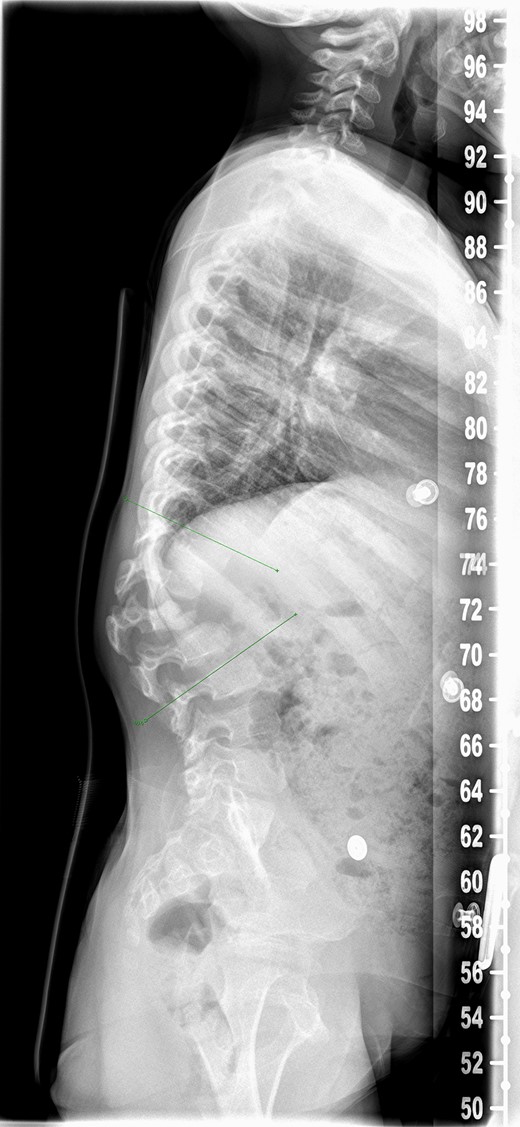
An 8-year-old girl with Mucopolysaccharidosis 1 and high lumbar kyphosis (Fig. 1) was admitted for posterior spinal fusion. Surgery was performed in prone position with multimodality spinal cord monitoring using a posterior midline approach. Spinal fixation was done with pedicle screws from T10 to L3 vertebrae. Spinal osteotomies were performed and kyphosis was corrected. It is the senior surgeon’s preference to augment fusion with i-Factor in order to promote early fusion in this patient group. Two 50 mm i-FactorTM bone graft Flex FR strips were cut along their length, one half was placed medial to the implants over the lamina and the other half placed lateral to the implants in posterolateral gutter. Allograft was placed on top of the strips. Wound was closed in layers leaving behind a drain over the fascia.
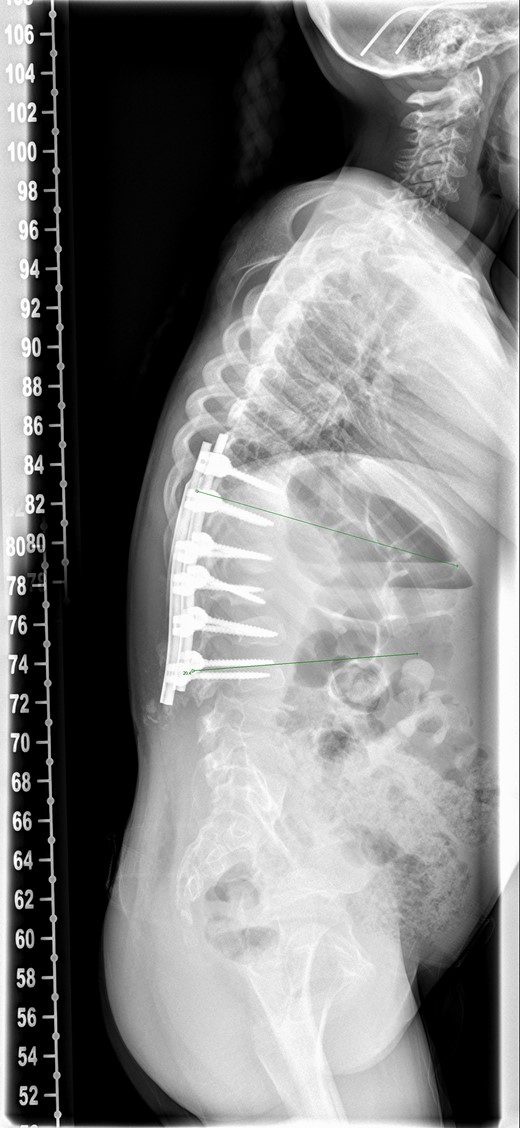
There was serous discharge with flakes of i-Factor in the drain postoperatively. Wound drain was removed on the second day and postoperative radiograph (Fig. 2) was satisfactory. Patient was discharged with a dry wound on the fifth day. Patient reattended hospital on seventh day with serous discharge from the drain site. There was no sign of infection and the wound was covered with a PICOTM dressing (Smith and Nephew), a negative pressure wound therapy system. At Day 10 the primary wound was healed, but serous fluid containing i-Factor flakes noted from the drain site. Patient was not started on antibiotics as there was no sign of infection and blood markers were normal. Wound was kept covered with a dressing and the discharge stopped.
Patient was reviewed 1 month after the surgery and radiographs (Fig. 3a and b) revealed heterotopic ossification in the paraspinal soft tissue. Matured mineralized bone was clearly seen near the distal end of the spinal construct. Wound was healed and there was no further discharge. No further intervention has been planned as patient remains asymptomatic with good radiographic correction of kyphosis.
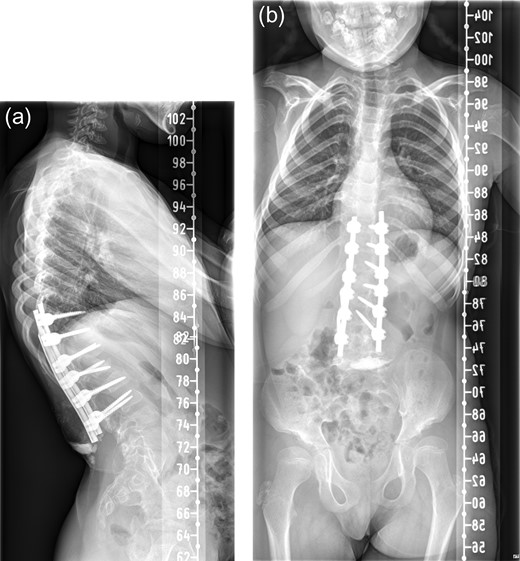
One month postoperative spine radiograph in (a) lateral view and (b) anteroposterior view showing heterotopic ossification.
DISCUSSION
Spinal fusion is a crucial step in correction of deformity to prevent failure of metalware and progression of deformity. Iliac crest bone grafting remains gold standard for fusion procedures. However, it is associated with complications such as donor site morbidity, increased surgical time and limited supply [4].
Peptide enhanced composite graft was granted permission in 2008 for use in Europe. There is a paucity of literature evidence to support its efficacy and safety. The few published studies of i-Factor [5–7] were on adults who had spinal surgery. There is no published literature regarding its complication profile in spinal surgery for children.
I-FactorTM is a composite made of anorganic bone matrix and bioactive P-15 peptide. The anorganic bone matrix is composed of calcium phosphate which provides osteoconductive properties of cell invasion and migration. The biologically active component is a synthetic 15 amino acid residue which when combined with the anorganic matrix provides a scaffold for cell invasion, binding and osteogenesis [5]. P-15 amino acid is similar to the one found in type 1 collagen of bone. Attachment of P-15 to osteogenic cells initiates a cascade of intracellular signalling that triggers the synthesis of extracellular matrix and growth factors. This induces cell proliferation, differentiation and subsequent osteogenesis [8]. It facilitates ingrowth of bone by promoting the migration of mesenchymal stem cells and other progenitor cells from surrounding tissue.
In a prospective study to assess efficacy of i-Factor in lumbar interbody fusion, it was observed that heterotopic ossification was noted in 48% who received i-Factor and 14% who received autograft [6]. This was attributed to graft migration outside the cage and disc space. I-Factor increases the opportunity of cell binding by making an abundance of P-15 available to osteogenic cells. After cell binding and proliferation the differentiation of cells increase TGFb-1, BMP-2 and BMP-7 influencing all processes of new bone formation [9]. Autograft and i-Factor have both proven to produce ectopic bone formation. The manufacturer (www.cerapedics.com) states that all cellular activity due to P-15 attachment is restricted to the implant surface thereby avoids ectopic bone growth. They also state that i-Factor is not a morphogen and the signals sent following P-15 attachment in a bony site activates cells that are pre-programmed to turn into osteoblasts. This was not true as use of i-Factor showed higher incidence of graft migration and ectopic bone formation when compared to autograft [6]. However, this did not affect the clinical outcome and was of little clinical significance.
BMP is another bone graft substitute which has been used in recent years for adult spinal surgery to augment fusion in lumbar and cervical spine. Evidence of symptomatic ectopic bone formation including neurological impairment have been reported following use of BMP [10]. BMP use has also shown to cause seroma formation due to exaggerated inflammatory reaction, with seroma fluid showing elevated levels of Interleukin-6 and Interleukin-8 [11]. We suspect that the serous discharge in our patient is a similar inflammatory response with i-Factor. BMP and i-Factor have similar mechanism of action and therefore can potentially have similar complication profile.
We have not planned any intervention to address the ectopic bone formation, as our patient remains asymptomatic and clinically well. Long term studies are required to assess the efficacy, safety and complications associated with the use of i-Factor in children. With this case report we document heterotopic ossification following spinal fusion with the use of i-Factor bone graft substitute.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- diagnostic radiologic examination
- genetic disorder
- child
- mucopolysaccharidosis i
- heterotopic ossification
- peptides
- spinal fusion
- surgical procedures, operative
- kyphosis
- acquired kyphosis
- congenital kyphosis
- spinal procedure
- neurologic deficits
- tissue scaffolds
- spinal fusion, posterior
- lumbar kyphosis



