-
PDF
- Split View
-
Views
-
Cite
Cite
Araya Zaesim, Viva Nguyen, Charles S Scarborough, Pure low-grade DCIS in a male patient: a case report, Journal of Surgical Case Reports, Volume 2018, Issue 5, May 2018, rjy109, https://doi.org/10.1093/jscr/rjy109
Close - Share Icon Share
Abstract
Breast cancer in males represents a small proportion of all cancers diagnosed. Pure ductal carcinoma in situ (DCIS), a low-grade form of breast cancer, is even more rare in male patients. We present a case of a 47-year-old male patient with a tender breast lump that was noted for 6 months. He was subsequently found to have a low grade, pure micropapillary and cribriform type DCIS with no evidence of invasive disease. Current literature does not provide distinct guidelines regarding management of male breast cancer, and it is currently managed in a similar fashion to female breast cancer.
INTRODUCTION
Male breast cancer is very rare and represents 1% of all cancers diagnosed in the USA [1]. Pure ductal carcinoma in situ (DCIS) in a male is even more rare and represents ~0.1% of all breast cancers [2]. Proposed risk factors for development of DCIS in men is the same as the risks factors for any male breast cancer and includes age, family history, exposure to radiation, conditions causing increased estrogen to androgen ratio (Klinefelter, obesity), and pathological gene mutations (CHEK2, BRCA2 > BRCA1) [3]. Management of male breast cancer stems from understanding of female breast cancer due to a lack of research in men and includes combinations of surgery, radiation and chemotherapy.
CASE REPORT
A 47-year-old Caucasian male with no family history of breast or ovarian cancer presented with a tender mass in the subareolar, outer left breast region. The patient noted the mass 6 months ago, but it had persisted with no improvement in symptoms. His past medical history includes gastric ulcer, gastroesophageal reflux, hypercholesterolemia, sciatica, sleep apnea and lumbar canal stenosis. He also previously had an appendectomy and a cholecystectomy as well as several sinus procedures. Social history includes being a social drinker, but he denies ever smoking. Physical exam showed symmetrical breasts with normal nipple–areolar complexes. No masses were palpated in the right breast, but there was a firm, tender, thickened area at the areolar border laterally in the left breast extending inferiorly ~2 cm. No nipple discharge or retraction was noted, and there were no palpable axillary or supraclavicular nodes bilaterally.
Bilateral mammogram demonstrated mild gynecomastia in both sides with no discrete mass or clusters of microcalcifications (Fig. 1). A follow-up ultrasound around the area of palpable concern did show a prominent duct with irregular contours at the 6:00 position in the left breast (Fig. 2). Excisional biopsy was recommended and a subareolar biopsy of the left breast was performed.
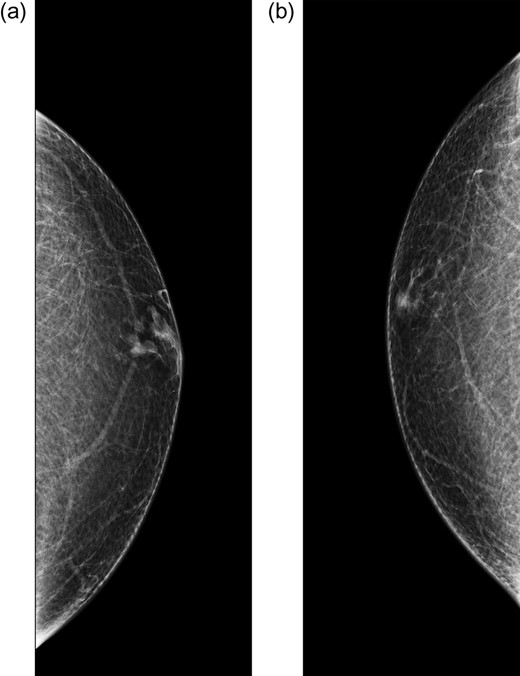
Diagnostic mammogram of the left (a) and right (b) breast demonstrates mild bilateral gynecomastia without discrete mass.
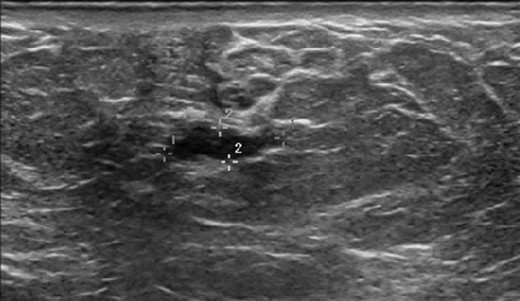
Ultrasound of the left breast at the 6:00 region reveals a mildly hypoechoic mass with ductal extension.
The pathology showed pure micropapillary and cribriform type low-grade DCIS with no evidence of invasive disease (Fig. 3). Breast specific gamma imaging (BSGI) and genetic testing for BRCA gene mutations were ordered. BRCA testing was negative for both BRCA1 and BRCA2 mutations. The BSGI revealed minimal parenchymal activity in the bilateral breasts, however, an area with vague radiotracer activity in a 2.5 cm diameter region was seen that was consistent with the biopsy location. After discussing the diagnosis and treatment options with the patient, he strongly desired bilateral mastectomy. After further discussions with other breast surgeons and a medical oncologist, simple left and right mastectomy with sentinel node biopsy on the left were offered to the patient.
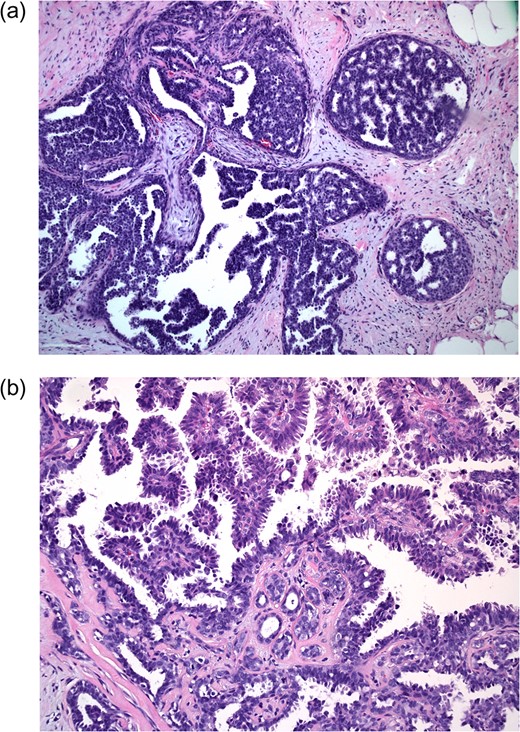
Excisional biopsy at 10× magnification (a) shows neoplastic cells within ducts; 20× magnification (b) demonstrates cribriform and micropapillary features (samples stained with hematoxylin and eosin).
Subsequently, a simple left mastectomy with sentinel lymph node biopsy and a simple right mastectomy were performed. The sentinel node was negative for metastatic carcinoma, and both breasts demonstrated no residual in situ or invasive carcinoma. The post-op course was uneventful, and the patient has continued medical follow-up with medical oncology.
DISCUSSION
DCIS is defined as a group of lesions caused by a proliferation of neoplastic cells within the mammary duct system of the breast [4]. It is differentiated from invasive breast cancer by detection of an intact basement membrane, but it is still associated with a risk for progression to invasive cancer, and thus, should be treated.
All breast cancer regardless of type is staged the same way in men as in women and follows the TNM staging protocol. Receptor expression for the hormones estrogen and progesterone as well as overexpression of the epidermal growth factor receptor-2 (HER2) is also regularly analyzed and plays a small role in the staging of breast cancers. For the case presented above, the tumor stage would be classified as a stage 0, low grade tumor under the latest guidelines by the American Joint Committee on Cancer [5].
Limited data exists regarding the natural history of low-grade DCIS. Low-grade DCIS does not often progress to invasive carcinoma with proper management. Research has shown that there is an increased likelihood of local recurrence with possible invasion after excisional biopsy even after 30 years, especially if the margins of excision were not fully negative [6]. In men, the incidence of bilateral invasive breast cancer is also extremely low. Thus, management for men focuses only on the breast where the primary lesion was detected. Patients should be educated about their risk for further evolution of their breast disease before providing them with options for management. Regardless of the risk, some patients still desire maximal risk reduction using techniques such as bilateral mastectomy, as in this case.
There are few studies regarding the management guidelines of breast cancer in males. This is likely due to the low incidence of breast cancer in the population. Male breast cancer is currently managed in a similar fashion to female breast cancer [7]. Even so, there are various options for management of such lesions in females. After confirmed biopsy of such a lesion, all management options revolve around surgery which is the primary method required to remove the lesion from the breast leaving negative margins. For a stage 0 low grade-DCIS, mastectomy (with or without reconstruction) is considered equivalent to breast conserving surgery (lumpectomy) with radiation [8, 9]. All options are offered to patients with discussions about risks and benefits as medical management involves patient satisfaction and cost.
Due to the rarity of male breast cancer, strong genetic predisposition is suspected whenever a case occurs. It is recommended that all male breast cancer patients should be tested for genetic mutations, especially BRCA1 and BRCA2 [10]. Men with BRCA2 mutations may have higher risk of developing breast cancer than men with BRCA1 mutations. Other gene mutations could also be considered in a workup and data shows that a multi gene panel test checking for alternative gene mutations could be offered.
CONFLICT OF INTEREST STATEMENT
None declared.



