-
PDF
- Split View
-
Views
-
Cite
Cite
Alessandro Moro, Mattia Todaro, Alessandro Pedicelli, Andrea Alexandre, Sandro Pelo, Piero Doneddu, Giulio Gasparini, Umberto Garagiola, Giuseppe D’Amato, Gianmarco Saponaro, Pseudoaneurysm of the internal maxillary artery secondary to subcondylar fracture: case report and literature review, Journal of Surgical Case Reports, Volume 2018, Issue 4, April 2018, rjy080, https://doi.org/10.1093/jscr/rjy080
Close - Share Icon Share
Abstract
Pseudoaneurysms are an uncommon complication of mandibular condylar-subcondylar fractures; however, if present, their recognition and management is mandatory to avoid life-threatening situations. The authors report a case of internal maxillary artery pseudoaneurysm rupture that occurred after an open reduction and internal fixation of a mandibular subcondylar fracture, along with a review of the literature.
INTRODUCTION
The authors describe a case of maxillary artery pseudoaneurysm (PA) haemorrhage that occurred during surgical reduction of a subcondylar mandibular fracture.
The bleeding was successfully managed with endovascular embolization.
The complications of the condylar fracture include pain, restricted mandibular movement, muscle spasm, deviation of the mandible, malocclusion, pathological changes in the temporomandibular junction, osteonecrosis, facial asymmetry, ankylosis, damage to cranial nerves, vascular injury, bleeding, growth disturbance, arteriovenous fistula and alteration of the balance in the masticatory muscles [1].
PAs are among the rarer complications of maxillofacial trauma. When encountered, they could cause life-threatening haemorrhaging.
This paper presents the case of a patient diagnosed with a subcondylar fracture with an underlying silent PA of the maxillary artery. Massive bleeding occurred during surgery and was successfully managed by selective catheterization of the internal maxillary artery and embolization with coils.
CLINICAL REPORT
A 55-year-old female patient was admitted to our facility after an inadvertent fall.
At the admission, the patient underwent a CT scan, the left mandibular subcondylar fracture was diagnosed (Fig. 1) and open reduction and rigid fixation was programmed.
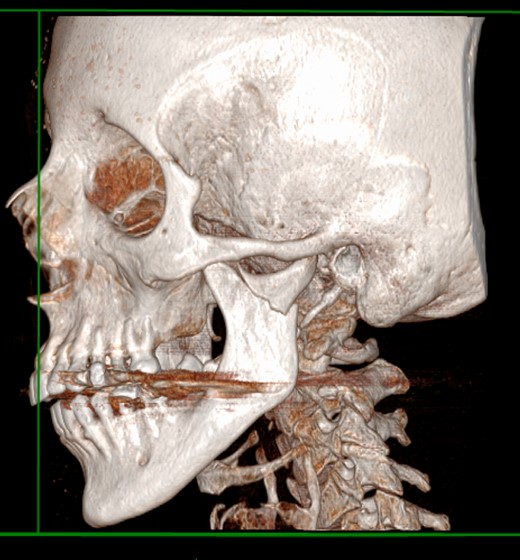
The mandible midline was deviated to the left side, and a right open bite was present, resulting in malocclusion.
The examination did not show any physical signs suggestive of PAs such as the presence of a solitary, compressible, pulsatile, nontender mass, neighbouring nerve compression symptoms [2] or a typical systolic bruit during auscultation.
The patient’s haemoglobin concentration was 12.4 g/dl with a haematocrit of 43.6%.
The patient underwent surgery two days after the admission; reduction and internal fixation of the mandible fracture was performed with a three hole titanium miniplate through intraoral access. The screws were placed through a minimal transbuccal approach.
Massive bleeding occurred just after the rigid fixation was performed. Haemostasis was attempted via the direct intraoral approach (cauterization-ligation), but the origin of the bleeding could not be visualized; therefore, surgeons tried to control the bleeding via a minimal retromandibular approach; a large retromandibular vein was detected and ligated without results. To avoid the complications of performing a larger approach, the surgeons decided to alert the interventional radiologist.
Total blood loss was estimated to be ~1600 ml during this period and patient’s haemoglobin concentration dropped to 7.4 g/dl.
A gauze was packed in the surgical wound in order to serve as compressive dressing for temporary bleeding control.
The patient was then transfused with one unit of fresh frozen plasma and two units of erythrocytes.
Intraoperative angiography was performed and revealed a PA in relation to the internal maxillary artery, ~1 cm posterior to the left subcondylar region (Fig. 2).
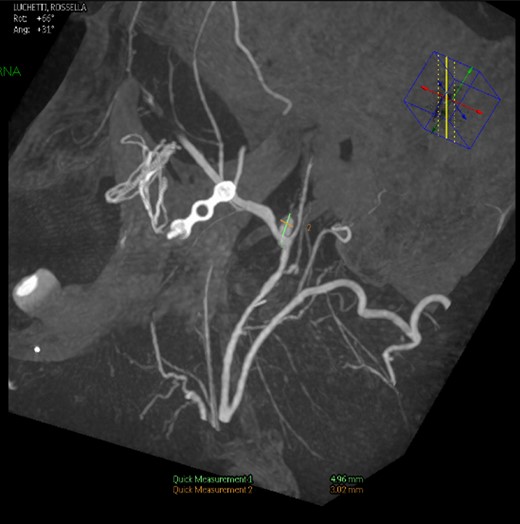
Left common carotid artery angiography in lateral views shows the PA (4.9 × 3 mm).
The patient was immediately sent to the interventional radiology unit.
Under general anaesthesia, the right femoral artery was punctured and a guide catheter (5-Fr) was inserted. Selective runs were performed at the left common carotid artery and external carotid artery. The PA was detected at the bifurcation of superficial temporal artery and internal maxillary artery. Through a selective microcatheterization (1.7-Fr), coiling of the lesion and the bifurcation was performed. The final angiograms showed complete exclusion of the PA after coiling, with collateral flow networks that maintained distal patency of the proximally occluded vessels (Fig. 3).
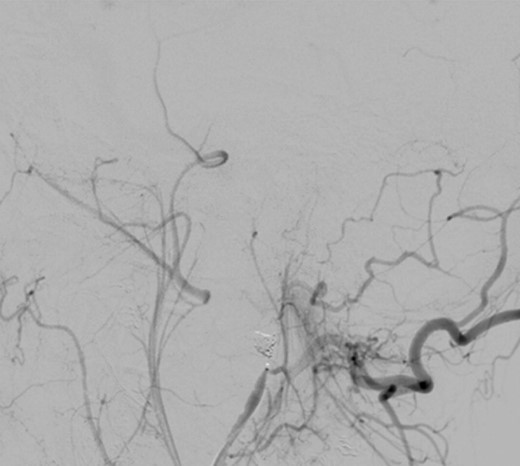
The patient was discharged on the third postoperative day; follow-up times were regular and at present, 3 months post-trauma, she has recovered completely with normal jaw function and occlusion.
DISCUSSION
Condylar process fracture is one of the most common mandibular fractures. It represents ~17.5–50% of all mandibular fractures [3].
The treatment of mandibular condyle fractures is controversial; conservative management, open reduction and internal fixation or external rigid fixation are options that the surgeon must consider case by case [4].
As mentioned above, complications are various.
PA (false aneurysm) secondary to mandibular condylar fracture, though rare, can cause significant morbidity.
PAs, or traumatic aneurysms, are different from true aneurysms because they involve the disruption of the inner layers of an artery after blunt, penetrating or even surgical trauma [5].
Other causes of PA are inflammation, infection, vasculitis, tumour and arteriosclerosis, also cases of unknown origins are reported in the literature [6].
The three branches of the external carotid system, which are the most frequent sites of PAs, are the superficial temporal, facial and maxillary artery [7].
Maxillary artery PAs have been reported following mandibular subcondylar osteotomies [8].
Orbay et al. [9] found that the mean distance of the maxillary artery to the medial border of the subcondylar portion of the mandible was 6.8 mm, while the mean distance of the maxillary artery to the tragal pointer was 16.2 mm in the horizontal plane and 21.4 mm in the vertical plane, with significant intra- and interindividual variations.
To the best of our knowledge, nine reports on condylar or subcondylar fracture-related PAs are reported in the last 10 years of literature (Table 1).
Condylar and subcondylar fracture-related PAs reported in the last 10 years of literature
| Author and year . | Artery involved . | Clinical manifestations . | Diagnostic method . | Timing . | Treatment . | PA size . |
|---|---|---|---|---|---|---|
| Bozkurt et al. [12] | Maxillary artery | Pulsatile mass | Angiography | Hours | Surgical treatment | n.d. (~10 cm) |
| El et al. [13] | External carotid artery | Pulsatile mass | CT angiography | 9 days | Endovascular embolization | 6 × 4 mm |
| Thakkur et al. [14] | Maxillary artery | Swelling | CT angiography | 5 weeks | Surgical treatment | 6, 6 × 4, 9 × 6, 9 cm |
| Chakrabarty et al. [15] | Internal maxillary artery | Pulsatile mass | Angiography | 2 weeks | Surgical treatment | 6 × 4 cm |
| Mohanty et al. [16] | Maxillary artery | Swelling | CT angiography | 4 weeks | Endovascular embolization and surgical treatment | 1, 5 × 2 cm |
| Li et al. [17] | Superficial temporal artery | Pulsatile mass | Angiography | 40 days | Endovascular embolization and surgical treatment | n.d. |
| Soh et al. [18] | Maxillary artery | Swelling | CT | 3 hours | Endovascular embolization | n.d. (~1 cm) |
| Katakol and Govindaraj [19] | Maxillary artery | Pulsatile mass, audible bruit | CT angiography | 2 months | Surgical treatment | n.d. (~2 cm) |
| Ribeiro et al. [20] | External carotid artery | Swelling | CT angiography | 4 weeks | Surgical treatment | n.d. |
| Author and year . | Artery involved . | Clinical manifestations . | Diagnostic method . | Timing . | Treatment . | PA size . |
|---|---|---|---|---|---|---|
| Bozkurt et al. [12] | Maxillary artery | Pulsatile mass | Angiography | Hours | Surgical treatment | n.d. (~10 cm) |
| El et al. [13] | External carotid artery | Pulsatile mass | CT angiography | 9 days | Endovascular embolization | 6 × 4 mm |
| Thakkur et al. [14] | Maxillary artery | Swelling | CT angiography | 5 weeks | Surgical treatment | 6, 6 × 4, 9 × 6, 9 cm |
| Chakrabarty et al. [15] | Internal maxillary artery | Pulsatile mass | Angiography | 2 weeks | Surgical treatment | 6 × 4 cm |
| Mohanty et al. [16] | Maxillary artery | Swelling | CT angiography | 4 weeks | Endovascular embolization and surgical treatment | 1, 5 × 2 cm |
| Li et al. [17] | Superficial temporal artery | Pulsatile mass | Angiography | 40 days | Endovascular embolization and surgical treatment | n.d. |
| Soh et al. [18] | Maxillary artery | Swelling | CT | 3 hours | Endovascular embolization | n.d. (~1 cm) |
| Katakol and Govindaraj [19] | Maxillary artery | Pulsatile mass, audible bruit | CT angiography | 2 months | Surgical treatment | n.d. (~2 cm) |
| Ribeiro et al. [20] | External carotid artery | Swelling | CT angiography | 4 weeks | Surgical treatment | n.d. |
Condylar and subcondylar fracture-related PAs reported in the last 10 years of literature
| Author and year . | Artery involved . | Clinical manifestations . | Diagnostic method . | Timing . | Treatment . | PA size . |
|---|---|---|---|---|---|---|
| Bozkurt et al. [12] | Maxillary artery | Pulsatile mass | Angiography | Hours | Surgical treatment | n.d. (~10 cm) |
| El et al. [13] | External carotid artery | Pulsatile mass | CT angiography | 9 days | Endovascular embolization | 6 × 4 mm |
| Thakkur et al. [14] | Maxillary artery | Swelling | CT angiography | 5 weeks | Surgical treatment | 6, 6 × 4, 9 × 6, 9 cm |
| Chakrabarty et al. [15] | Internal maxillary artery | Pulsatile mass | Angiography | 2 weeks | Surgical treatment | 6 × 4 cm |
| Mohanty et al. [16] | Maxillary artery | Swelling | CT angiography | 4 weeks | Endovascular embolization and surgical treatment | 1, 5 × 2 cm |
| Li et al. [17] | Superficial temporal artery | Pulsatile mass | Angiography | 40 days | Endovascular embolization and surgical treatment | n.d. |
| Soh et al. [18] | Maxillary artery | Swelling | CT | 3 hours | Endovascular embolization | n.d. (~1 cm) |
| Katakol and Govindaraj [19] | Maxillary artery | Pulsatile mass, audible bruit | CT angiography | 2 months | Surgical treatment | n.d. (~2 cm) |
| Ribeiro et al. [20] | External carotid artery | Swelling | CT angiography | 4 weeks | Surgical treatment | n.d. |
| Author and year . | Artery involved . | Clinical manifestations . | Diagnostic method . | Timing . | Treatment . | PA size . |
|---|---|---|---|---|---|---|
| Bozkurt et al. [12] | Maxillary artery | Pulsatile mass | Angiography | Hours | Surgical treatment | n.d. (~10 cm) |
| El et al. [13] | External carotid artery | Pulsatile mass | CT angiography | 9 days | Endovascular embolization | 6 × 4 mm |
| Thakkur et al. [14] | Maxillary artery | Swelling | CT angiography | 5 weeks | Surgical treatment | 6, 6 × 4, 9 × 6, 9 cm |
| Chakrabarty et al. [15] | Internal maxillary artery | Pulsatile mass | Angiography | 2 weeks | Surgical treatment | 6 × 4 cm |
| Mohanty et al. [16] | Maxillary artery | Swelling | CT angiography | 4 weeks | Endovascular embolization and surgical treatment | 1, 5 × 2 cm |
| Li et al. [17] | Superficial temporal artery | Pulsatile mass | Angiography | 40 days | Endovascular embolization and surgical treatment | n.d. |
| Soh et al. [18] | Maxillary artery | Swelling | CT | 3 hours | Endovascular embolization | n.d. (~1 cm) |
| Katakol and Govindaraj [19] | Maxillary artery | Pulsatile mass, audible bruit | CT angiography | 2 months | Surgical treatment | n.d. (~2 cm) |
| Ribeiro et al. [20] | External carotid artery | Swelling | CT angiography | 4 weeks | Surgical treatment | n.d. |
The mean time of PA diagnosis was 24 days (range: 3 hours–2 months) from the traumatic event.
The clinical manifestations of PA are variable, including a pulsatile mass, craniocervical pain, bleeding, dysphagia, hoarseness and neurologic deficits [11].
Contrast-enhanced CT and catheter angiography are the gold standard in diagnosing PA of the IMA.
The absence of pulsations does not rule out the presence of a PA, in fact, the clinical manifestation features were swelling and mass effect of the surrounding tissue in all reports; only in five cases [12, 13, 15, 17, 19] pulsatile swelling and bruit on auscultation were detected.
In two cases [16, 20], the presence of persistent swelling 1 month after the traumatic event led to the incorrect diagnosis of infection, so incision and drainage were attempted, and in both cases massive haemorrhage occurred.
In six cases, the diagnosis was performed only by CT angiography, between these in one case [14] the surgeons misinterpreted the PA to be arising from the superficial temporal artery while the feeding vessel was the maxillary artery.
In seven clinical reports, the artery involved was the internal maxillary secondary to condylar fracture, in two cases the external carotid artery secondary to subcondylar fracture and one case the superficial temporal artery secondary to a condylar fracture.
Due to the close anatomical relationship between the external carotid artery (ECA) and its branches and the mandible, vascular injuries and subsequent PA may be underdiagnosed and dismissed as ‘fracture haematoma’ [10].
Five cases underwent surgical treatment, two cases endovascular embolization and in two cases both surgical and endovascular treatment was performed.
PA management includes different surgical and endovascular options.
The risk of life-threatening haemorrhage following rupture is high; in such cases the control of haemorrhage is challenging.
Surgical ligation of the origin of the PA feeding vessels is the best method of haemostasis but is technically demanding.
The preauricular incision offers limited access to the vessel and a higher risk of facial nerve injury.
Ligation of the external carotid artery or its distal branches may be considered, but remains a controversial manoeuvre [20].
Yin showed the difficulty in achieving haemostasis because of the need to perform multiple ligatures to reduce blood flow [21].
A rich network of collateral arterial anastomoses exists with the contralateral external carotid artery branches, the ipsilateral internal carotid artery via the ophthalmic artery, the ipsilateral subclavian artery via the costocervical and thyrocervical trunks, and the ipsilateral vertebral artery. As described by Rosenberg et al. [22] unilateral ligation of the external carotid artery below the level of the lingual and facial arteries leads only to a partial (40%) decrease in the maxillary artery blood flow.
Abraham et al. [23] documented an increased internal carotid artery (ICA) perfusion after ECA ligation, presumably a result of increasing ICA perfusion pressure.
In hospitals where interventional radiology is available, angiographic embolization must be considered if a PA is suspected.
Embolization in the head and neck region is a well-known therapy in the management of epistaxis [24], post-traumatic haemorrhage [25], presurgical tumour devascularization [26] and aneurysm/PA occlusion [27, 28].
Acute complications of endovascular treatment include distal thromboembolic events (occlusion of the central retinal artery, ischaemic stroke due to potential anastomosis between the IMA and the ophthalmic artery and local tissue ischaemia). Other reported complications are perforations, glued vein, microcatheter fracture, and vessel dissection or branch occlusion [11, 18, 29, 30].
Embolization with liquid embolic agents can be dangerous in patients with significant anastomosis with neighbouring major vessels. Endovascular procedures may be challenging in case of severe atheromatous disease, and allergy to any of the contrast or embolizing agents represents an absolute contraindication. There are numerous embolising agents used such as gel foam, isobutylcyanoacrylate, balloons and coils.
In trained hands, morbidity associated with such treatment is almost negligible (0.03% mortality and 1.73% morbidity) and results are promising [31].
In the cases reported above where endovascular treatment was performed, no complication was reported.
In one case treated with surgical excision of the PA, facial nerve deficit was documented [15].
CONCLUSION
Despite the close anatomic relationship between the mandibular condyle and the external carotid artery or its terminal branch, the maxillary artery vascular lesions associated with condylar fractures are uncommon, although such lesions can lead to life-threatening situations.
The surgeon should always keep in mind the potential presence of a silent PA even if the classic signs or symptoms are not evident, as in our case.
When massive bleeding occurs during surgery, suspect signs appear before or after surgery (even up to 60 days [19]), the presence of a PA must be considered.
The direct ligation of PA feeding vessels is time consuming, technically demanding and not always effective.
ECA ligation, a relatively simple procedure, not always allows complete haemostasis in case of PA.
We believe that the role of interventional radiology is crucial for the diagnosis and treatment of traumatic PA; endovascular embolization is a safe and effective procedure that avoids the morbidity of an open surgical treatment.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.



