-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher M Dress, Elisabet K Tassis, A case of Dupuytren’s disease managed with viable cryopreserved placental membrane adjunct to open palmar fasciectomy, Journal of Surgical Case Reports, Volume 2018, Issue 3, March 2018, rjy055, https://doi.org/10.1093/jscr/rjy055
Close - Share Icon Share
Abstract
Dupuytren’s disease (DD) is a rare connective tissue disorder resulting in progressive fibrosis and thickening of the palmar fascia, and contracture of the fingers due to excessive collagen deposition. Staged surgical interventions are reserved for severe cases, yet worsening of fibrosis and contracture of fingers post-surgery, has been reported to have a recurrence rate as high as 85%. Here, the authors report on use of viable cryopreserved placental membrane (vCPM) allograft as an adjunct to open fasciectomy. In a patient with debilitating bilateral DD contractures of >20 years duration, this novel approach resulted in a 34.8% range of motion (ROM) improvement and ability to fully extend all digits of the right hand. No adverse events were recorded. At 1 year post-surgery, the patient has no decrease in ROM. Results indicate that vCPM incorporation in open fasciectomy may provide benefit in reducing contracture recurrence in DD patients.
INTRODUCTION
Dupuytren’s disease (DD) is a connective tissue disorder attributed to overstimulation of fibroblast proliferation resulting in excessive collagen deposition and thickening of the palmar fascia. Characterized by the development of abnormal longitudinal fibrous tissues and contracted cords extending into palmar bands, DD causes permanent joint flexion, stiffness, decreased range-of-motion (ROM) and pain. Over time digital flexion deformities worsen, most commonly over metacarpophalangeal (MCP) and proximal interphalangeal joints, and patients cannot fully extend and utilize digits [1].
Staged percutaneous and open palmar fasciectomy (OPF) are often reserved for severe cases of bilateral DD or >1-digit involvement [1]. Surgical intervention success varies and risks include contracture and scar formation, nerve injury, complex regional pain syndrome, infection or partial to total loss of affected digits with recurrence risks reported anywhere from 20.9 to 84.9% [2, 3].
Here, the authors report on a debilitating case of DD contractures of >20 years duration managed using staged OPF and viable placental allograft-MCP joint overlay with the aim of reducing post-operative (post-op) contracture reformation.
CASE REPORT
A 72-year-old right hand-dominant Caucasian female presented with history of anemia, osteoarthritis, type 2 diabetes mellitus, hypertension, congestive heart failure, hyperlipidemia and >20 years duration of bilateral hand contractures.
Hand deformities at the base of digits were visually noted and confirmed via radiographic images. Assessment revealed diffuse swelling, joint instability with subluxation, and generalized weakness/tenderness of the hands. Cords were palpated over palmar surfaces of the right fourth and fifth metacarpals and left fourth metacarpal. Right palmar contractures were more severe than the left, with right digit ROM extending from 30° to 82.5° (Fig. 1A) with inability to fully flex all digits (Fig. 1B). Limited ROM prevented normal activities (writing, dressing, bathing, grooming and gripping objects, i.e. cane). Daily ongoing pain score of 8/10 was characterized as intermittent, sharp, stabbing and aching. Discomfort was aggravated by grasping, lifting, carrying and reaching and could be alleviated with rest. Multiple years of failed rehabilitative therapy led to the decision for staged contracture removal through OPF, beginning with the dominant and more symptomatic right hand.
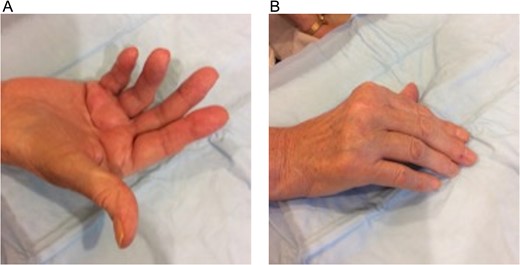
Dupuytren’s disease patient with bilateral palmar fibromatosis of >20 years duration. Inflamed palpable chords over the fourth and fifth metacarpal bones of the right-hand shows: (A) collective average active range of motion deficit of 40° in all digits (average range 30°–82.5°) and (B) inability to fully extend the digits.
Under intraoperative loupe magnification, a Brunner-type incision was made over the right fourth metacarpal palmar surface. Dissection proceeded bluntly using scissors to raise and secure skin flaps in an open retracted fashion with simple interrupted 3-0 nylon sutures, allowing for visualization of the fibrous cord (Fig. 2A). Digital neurovascular bundles over the fourth metacarpal bone were identified and dissected away from the posterior deep cord surface and folded up over the metacarpal. The cord was divided proximally and dissected along the superficial surface and lateral borders to its distal terminus at the MCP joint. Ulnar-directed lateral cord extensions to the fifth metacarpal bone were isolated and dissected away. A separate and individual contracture over the fifth metacarpal was not identified. The excised 4.5 × 1.5 × 0.5 cm3 fibrous cord (dense tan-pink rubbery tissue with adherent yellow adipose) was collected in formalin for pathologic examination (Fig. 2B). The wound was irrigated and bleeding vessels cauterized with bipolar Bovie electrocauter. A 5 × 5 cm2 viable cryopreserved placental membrane (vCPM) (Fig. 3A) was prepared per manufacturer’s instructions and placed proximally in the dissection space as direct MCP joint overlay (Fig. 3B). Skin incision was closed with simple interrupted 3-0 nylon sutures and dressed with XeroformTM followed by bulky hand gauze and 4-inch Ace-wrap. Patient was discharged home same-day with pain medications and instructed to keep dressing clean and intact.
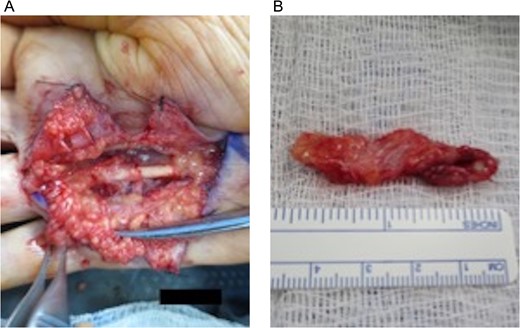
Open fasciectomy intraoperative images: (A) Raised and secured skin flaps revealing visible fibrotic contract cord overlaying the fourth metacarpal bone and (B) excised fibrotic palmar fascia specimen measuring 4.5 × 1.5 × 0.5 cm3.
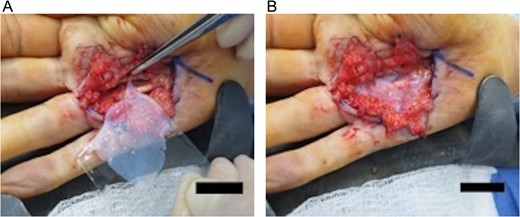
(A) The application of a 5 × 5 cm2 viable cryopreserved placental membrane. (B) The placental graft is placed proximally in the surgical dissection space overlying the metacarpophalangeal joint.
On post-op Day 7, the right palmar incision was clean, dry and intact without evidence of erythema, tenderness, fluid collection or drainage. Improved digit motion with a pain score of 2/10 was reported. On post-op Day 21, sutures were removed, pain score decreased to 0/10 and patient was prescribed occupational therapy (OT) (22 sessions/51 days) consisting of neuromuscular re-education, cold laser, manual therapeutic exercise programs for joint and soft-tissue mobility, ROM, soft-tissue extensibility, motor control and strengthening.
On post-op Day 63, hypertrophic scar formation at the distal portion of the incision site (area receiving no intra-op vCPM) was treated with sub-dermal 0.5 ml triamcinolone (Kenalog-10) injection. By post-op Day 76, scar thickness was reduced with patient demonstrating improved grip strength. Collective improvement in active-ROM values for all right-hand digits equaled 34.8% (58.3% start of OT ROM; 93.1% OT discharge ROM). Patient was now able to extend digits to independently bathe, groom and dress herself using her right extremity (Fig. 4). At 1 year post-surgery, the surgical scar remained smooth without density or contracture. Patient has experienced no treatment-related adverse events and shown no regression of rehabilitated-ROM.
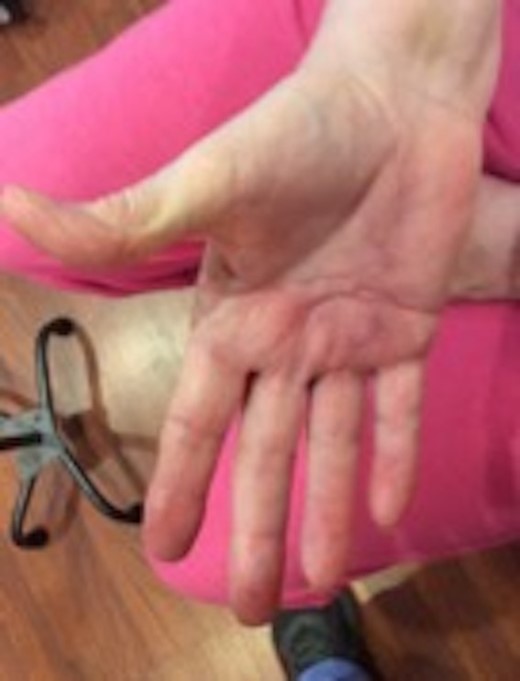
Post-operative follow-up demonstrating significant increase in range of motion (average range: 6.25°–90°) demonstrated by full extension of all digits in the right hand.
DISCUSSION
Human placental membrane (HPM) use was initially reported in burn management [4]. Recent advances in tissue preservation technology have allowed for commercialization and use of viable placental products to expand to surgical settings. vCPM, processed with aseptic cryopreservation technology, retains native HPM components—a structural 3-D extracellular matrix, resident growth factors and cytokines, and viable cells, including mesenchymal stem cells [5]. This point-of-care allograft retains its structure after thawing.
Here, vCPM was applied proximally in the surgical dissection space over the MCP joint with the goal of preventing contracture reoccurrence. Previously reported data showed that vCPM application to a hand burn resulted in absence of contracture with ROM restoration and absence of scar reformation when used as an adjunct to chronic keloid resection [6, 7]. Notably in this DD patient, the distal part of the surgical incision without vCPM underlay required triamcinolone injection due to post-op fascia thickening. To date, the patient has not experienced contracture reformation or reduced ROM. No adverse events were recorded, including infection—a common complication occurring in up to 18% cases post OPF [3]. Pain reduction of 8/10 to 0/10 led to reduced use of narcotics post-surgery, a phenomenon also reported in burn hand cases treated with vCPM [6].
These outcomes support vCPM use as a potential adjunct to OPF for treatment of DD or other surgical interventions known for high-risk post-op fibrosis or contractures. The authors recognize that despite initial positive clinical outcomes, long-term follow-up will provide additional insight on the utilization and clinical outcomes of vCPM in surgical fasciectomies.
CONFLICT OF INTEREST STATEMENT
E.K.T. is a full-time employee of Osiris Therapeutics, Inc. (‘Osiris’). C.M.D. is currently a paid consultant and a speaker for Osiris; however, C.M.D. received no compensation or incentives for the performance and publication of this case.
FUNDING
None.
ETHICS APPROVAL/INSTITUTIONAL REVIEW BOARD (IRB)
Not required.



