-
PDF
- Split View
-
Views
-
Cite
Cite
Timothy J Harris, William C Beck, Avi Bhavaraju, Benjamin Davis, Mary K Kimbrough, Joseph C Jensen, Anna Privratsky, John R Taylor, Kevin W Sexton, Severe acute gallstone pancreatitis with diffuse hemorrhagic gastritis, Journal of Surgical Case Reports, Volume 2018, Issue 3, March 2018, rjy048, https://doi.org/10.1093/jscr/rjy048
Close - Share Icon Share
Abstract
A 67-year-old male presented with acute pancreatitis secondary to gallstones, also known as acute biliary pancreatitis, and subsequently developed gastric outlet obstruction and was transferred to our hospital. A gastro-jejunal feeding tube was placed and an open cholecystectomy was performed. The patient had a pancreatic drain placed for interval increase in pancreatic necrosis and then nearly exsanguinated from gastroduodenal artery pseudoaneurysm bleed. This was managed by coiling the gastroduodenal artery. The patient underwent a pancreatic necrosectomy with malencot drain placement and developed a post-operative upper gastrointestinal bleeding. An EGD showed diffuse gastritis, but no varices. And 18 days later the patient rebled, with the same diffuse gastritis. After further complications the patient elected to receive palliative care at a hospice facility. We are presenting this unusual case of diffuse, hemorrhagic gastritis after acute necrotizing pancreatitis.
INTRODUCTION
A 2012 study reviewing the prevalence and impact of gastrointestinal (GI) diseases in the USA, found that acute pancreatitis (AP) was the most commonly diagnosed GI disease requiring hospitalization [1]. AP also had the highest total cost at $2599, 686 000. Gallstones are the leading cause of AP accounting for 35–40% of cases worldwide [2]. Gallstones that become lodged in the ampulla of Vater cause AP by blocking the flow of bile and pancreatic enzymes into the duodenum [3]. This induces a hyperstimulation of the acinar cells of the pancreas to increase production of activated enzymes.
AP is categorized by the revised Atlanta classification (Table 1), as mild, moderately severe or severe based on the presence of organ failure, transient or persistent, and/or local or systemic complications [4]. Mild AP has no organ failure or local or systemic complications. Moderately severe AP is characterized by transient organ failure (lasting <48 h) and/or local or systemic complications without persistent organ failure. Severe AP includes those cases where single or multiple organ failure lasts for >48 h (persistent organ failure).
| Mild | No organ failure and no local or systemic complications |
| Moderately severe | Transient organ failure (<48 h) and/or local or systemic complications without persistent organ failure (>48 h) |
| Severe | Persistent organ failure (>48 h):* single organ failure or multiple organ failure |
| Mild | No organ failure and no local or systemic complications |
| Moderately severe | Transient organ failure (<48 h) and/or local or systemic complications without persistent organ failure (>48 h) |
| Severe | Persistent organ failure (>48 h):* single organ failure or multiple organ failure |
*Persistent organ failure is defined by a modified Marshall score (Table 2).
| Mild | No organ failure and no local or systemic complications |
| Moderately severe | Transient organ failure (<48 h) and/or local or systemic complications without persistent organ failure (>48 h) |
| Severe | Persistent organ failure (>48 h):* single organ failure or multiple organ failure |
| Mild | No organ failure and no local or systemic complications |
| Moderately severe | Transient organ failure (<48 h) and/or local or systemic complications without persistent organ failure (>48 h) |
| Severe | Persistent organ failure (>48 h):* single organ failure or multiple organ failure |
*Persistent organ failure is defined by a modified Marshall score (Table 2).
The majority of patients with severe AP also have pancreatic necrosis and at least 30% risk of mortality [5]. Organ failure is characterized by the modified Marshall scoring system (Table 2) evaluating respiratory, cardiovascular and renal systems. Respiratory failure is defined as PaO2/FiO2 of 300 or less [4]. Cardiovascular failure is defined as systolic blood pressure <90 mmHg and not fluid responsive or systolic blood pressure <90 mmHg and a pH <7.3. Renal failure is defined as a serum creatinine of 1.9 mg/dl or higher.
| Organ system . | 0 . | 1 . | 2 . | 3 . | 4 . | |
|---|---|---|---|---|---|---|
| Respiratory (PaO2/FiO2) | >400 | 301–400 | 201–300 | 101–200 | <101 | |
| Renal* (Serum creatinine: μmol/l, [mg/dl]) | <134 [<1.4] | 134–169 [1.4–1.8] | 170–310 [1.9–3.6] | 311–439 [3.6–4.9] | >439 [>4.9] | |
| Cardiovascular (Systolic bp, mmHg)** | >90 | <90, Fluid responsive | <90, Not fluid responsive | <90, pH<7.3 | <90, pH<7.2 | |
| For non-ventilated patients, the FiO2 can be estimated from below | ||||||
| Supplemental oxygen (l/min) | FiO2 (%) | |||||
| Room air | 21 | |||||
| 2 | 25 | |||||
| 4 | 30 | |||||
| 6–8 | 40 | |||||
| 9–10 | 50 | |||||
| Organ system . | 0 . | 1 . | 2 . | 3 . | 4 . | |
|---|---|---|---|---|---|---|
| Respiratory (PaO2/FiO2) | >400 | 301–400 | 201–300 | 101–200 | <101 | |
| Renal* (Serum creatinine: μmol/l, [mg/dl]) | <134 [<1.4] | 134–169 [1.4–1.8] | 170–310 [1.9–3.6] | 311–439 [3.6–4.9] | >439 [>4.9] | |
| Cardiovascular (Systolic bp, mmHg)** | >90 | <90, Fluid responsive | <90, Not fluid responsive | <90, pH<7.3 | <90, pH<7.2 | |
| For non-ventilated patients, the FiO2 can be estimated from below | ||||||
| Supplemental oxygen (l/min) | FiO2 (%) | |||||
| Room air | 21 | |||||
| 2 | 25 | |||||
| 4 | 30 | |||||
| 6–8 | 40 | |||||
| 9–10 | 50 | |||||
*A score for patients with pre-existing chronic renal failure depends on the extent of further deterioration of baseline renal function. No formal correction exists for a baseline serum creatinine >134 μmol/l or >1.4 mg/dl.
**Off inotropic support.
A score of 2 or more in any system defines the presence of organ failure.
| Organ system . | 0 . | 1 . | 2 . | 3 . | 4 . | |
|---|---|---|---|---|---|---|
| Respiratory (PaO2/FiO2) | >400 | 301–400 | 201–300 | 101–200 | <101 | |
| Renal* (Serum creatinine: μmol/l, [mg/dl]) | <134 [<1.4] | 134–169 [1.4–1.8] | 170–310 [1.9–3.6] | 311–439 [3.6–4.9] | >439 [>4.9] | |
| Cardiovascular (Systolic bp, mmHg)** | >90 | <90, Fluid responsive | <90, Not fluid responsive | <90, pH<7.3 | <90, pH<7.2 | |
| For non-ventilated patients, the FiO2 can be estimated from below | ||||||
| Supplemental oxygen (l/min) | FiO2 (%) | |||||
| Room air | 21 | |||||
| 2 | 25 | |||||
| 4 | 30 | |||||
| 6–8 | 40 | |||||
| 9–10 | 50 | |||||
| Organ system . | 0 . | 1 . | 2 . | 3 . | 4 . | |
|---|---|---|---|---|---|---|
| Respiratory (PaO2/FiO2) | >400 | 301–400 | 201–300 | 101–200 | <101 | |
| Renal* (Serum creatinine: μmol/l, [mg/dl]) | <134 [<1.4] | 134–169 [1.4–1.8] | 170–310 [1.9–3.6] | 311–439 [3.6–4.9] | >439 [>4.9] | |
| Cardiovascular (Systolic bp, mmHg)** | >90 | <90, Fluid responsive | <90, Not fluid responsive | <90, pH<7.3 | <90, pH<7.2 | |
| For non-ventilated patients, the FiO2 can be estimated from below | ||||||
| Supplemental oxygen (l/min) | FiO2 (%) | |||||
| Room air | 21 | |||||
| 2 | 25 | |||||
| 4 | 30 | |||||
| 6–8 | 40 | |||||
| 9–10 | 50 | |||||
*A score for patients with pre-existing chronic renal failure depends on the extent of further deterioration of baseline renal function. No formal correction exists for a baseline serum creatinine >134 μmol/l or >1.4 mg/dl.
**Off inotropic support.
A score of 2 or more in any system defines the presence of organ failure.
CASE REPORT
A 67-year-old male presented to his local hospital with postprandial right upper quadrant (RUQ) abdominal pain and vomiting. CT scan of the abdomen and pelvis with oral contrast showed extensive stranding and ill-defined fluid attenuation surrounding his pancreas (Fig. 1). Initial serum amylase and serum lipase levels were 2918 and 17 360, respectively. He was diagnosed with acute, necrotizing, gallstone pancreatitis and developed gastric outlet obstruction (GOO) 9 days after the onset of his symptoms, at which time he was transferred to our institution for further care. On arrival, he had GOO and renal failure (creatinine 2.1 mg/dl). A gastro-jejunal (GJ) feeding tube was placed for jejunal feeds and gastric decompression. On hospital Day 7, the patient’s pain had resolved and a cholecystectomy was attempted laparoscopically, but was converted to open due to inflammation around the infundibulocystic junction. Subsequently, a follow-up contrasted CT showed marked interval necrosis of pancreatic tissue resulting in numerous lobulated soft tissue and gas collections in the upper abdomen, midline upper pelvis, and in the gallbladder fossa (Fig. 2). A pancreatic drain was placed by interventional radiology (IR) on hospital Day 20 for interval increase in pancreatic necrosis on CT. On hospital Day 23, IR embolization of gastroduodenal artery (GDA) was performed due to GI bleeding. Laparoscopic necrosectomy with malencot drain placement was performed on hospital Day 36. Post-operatively, he had a large upper GI bleed requiring emergent endotracheal intubation and initiation of the massive transfusion protocol. Angiography was performed and was negative. On the following day, hospital Day 39, GI performed an EGD and found hemorrhagic gastritis, but no localizable bleeding. He was placed on high dose PPI, carafate and octreotide. He progressed to acute renal failure with initiation of hemodialysis on hospital Day 45. On hospital Day 47, a follow-up EGD was done with exchange of the gastrojejunostomy tube, and hemorrhagic gastritis was still present. He was transferred to the general surgery inpatient unit the following day. On hospital Day 54, he developed another upper GI bleed requiring ICU transfer. EGD was again performed and the hemorrhagic gastritis remained, but no active site of bleeding was identified. On hospital Day 56, a second look EGD was done with similar findings of hemorrhagic gastritis with no identifiable active bleeding. On hospital Day 58, the patient elected to receive palliative care rather than undergo subtotal gastrectomy. He died 9 days later at home.
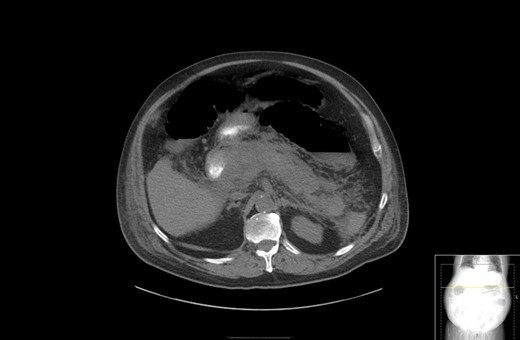
CT with oral contrast showing extensive stranding and ill-defined fluid attenuation surrounding his pancreas.
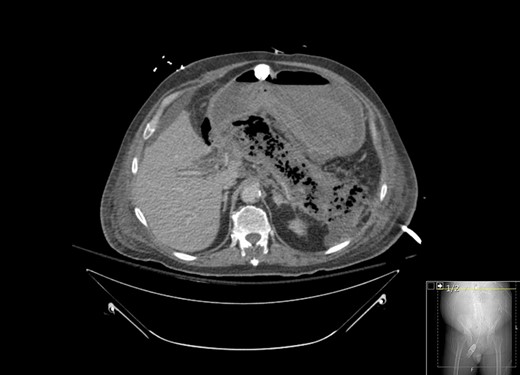
CT with contrast showing marked interval necrosis of pancreatic tissue.
DISCUSSION
The annual incidence of AP is on the rise. There was a 30% increase in the number of cases from 2000 to 2009 for which AP was the principal discharge diagnosis [1]. Additionally, there is no GI disease for which Americans are hospitalized more, or that costs Americans more, than AP [1]. In this case, we were faced with the rare complication of hemorrhagic gastritis following AP. After reviewing the literature, there are very few cases of hemorrhagic gastritis following AP.
We used multiple approaches in attempt to treat our patient’s gastric bleeding. First, IR embolized the GDA. We treated the patient with octreotide, and we performed multiple upper endoscopies in an attempt to locate a bleeding source. With each EGD we found no source of frank bleeding, only diffuse gastritis and clot formation. Given these findings, the patient was offered subtotal gastrectomy; however, the patient chose to go home with palliative care. To conclude, hemorrhagic gastritis is a rare and severe complication of pancreatitis with high morbidity and mortality. No best treatment strategy exists for this difficult complication.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This work was funded by the University of Arkansas for Medical Sciences Clinician Scientist Program.
REFERENCES
- pancreatitis, acute
- pseudoaneurysm
- hemorrhage
- biliary calculi
- gastritis
- hospice
- pancreatitis, necrotizing, acute
- varicosity
- gastric outlet obstruction
- jejunum
- palliative care
- pancreas
- feeding tubes
- upper gastrointestinal bleeding
- hemorrhagic gastritis
- pancreatitis, biliary
- cholecystectomy, open
- gallstone acute pancreatitis
- gastroduodenal artery
- pancreatic necrosis
- debridement of pancreatic and peripancreatic necrosis



