-
PDF
- Split View
-
Views
-
Cite
Cite
James McMahon, Aneesh Dave, Assad Zahid, Kirk Austin, Peritoneal encapsulation as a cause of chronic recurrent abdominal pain in a young male, Journal of Surgical Case Reports, Volume 2018, Issue 3, March 2018, rjy033, https://doi.org/10.1093/jscr/rjy033
Close - Share Icon Share
Abstract
This case report describes an otherwise well 20-year-old male who presented to hospital with vague, long-standing abdominal symptoms and was found to have peritoneal encapsulation.
INTRODUCTION
Congenital peritoneal encapsulation (CPE) is a poorly understood condition in which an accessory peritoneal layer encapsulates the majority of small bowel and a variable length of large bowel [1]. It is an extremely rare phenomenon which is most often discovered incidentally intra-operatively. However, it may also present with acute small bowel obstruction [2, 3] or, less commonly with non-specific chronic abdominal pain [4], as in this case. This report also includes a general discussion about the aetiology and the entity of conditions to which CPE belongs.
CASE REPORT
A 20-year-old male presented to a major hospital with a history of intermittent severe abdominal pain which commenced at approximately 7 years of age. The pain was characterized by a diffuse cramping quality, with greatest severity in the supra-pubic region, and was relatively stable throughout its course. In the days prior to this presentation, the pain had increased in frequency and severity. The patient had been previously investigated with ultrasonography, which yielded no significant abnormality. There was no significant past medical or surgical history and he was on no regular medications.
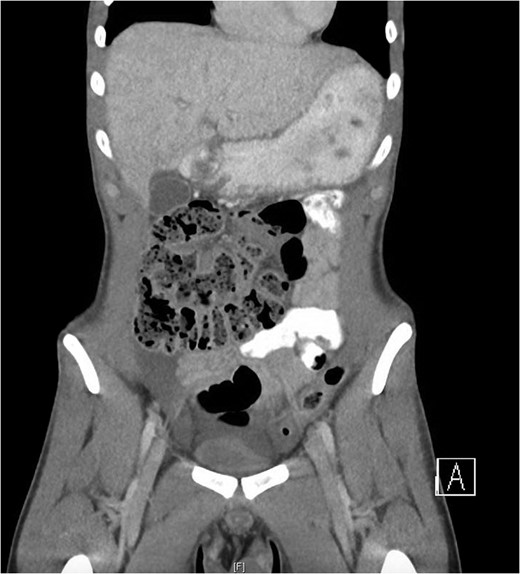
The only notable clinical finding was tenderness in the supra-pubic region. There was no evidence of peritonism. Routine blood tests were normal. Abdomino-pelvic computed tomography (Fig. 1) demonstrated a moderate amount of free fluid on the right side of the abdominal cavity and appendiceal thickening. A testicular ultrasound yielded no significant abnormality. Due largely to the unexplained CT findings, the patient was consented for a diagnostic laparoscopy.
Laparoscopy revealed an accessory intra-abdominal cyst encapsulating the entire length of small bowel (Fig. 2) and the large bowel to the mid-transverse colon.
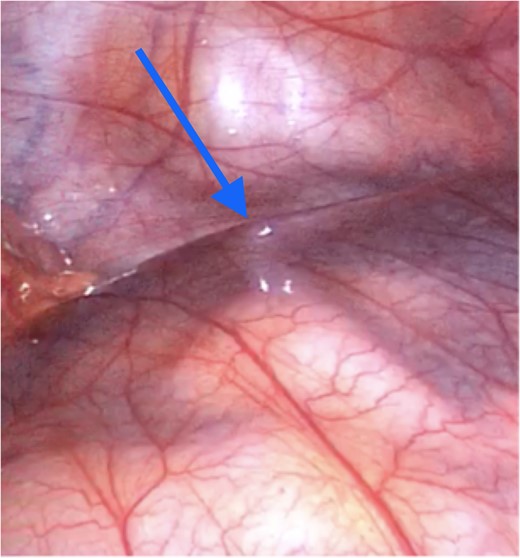
Peritoneal encapsulation cyst with small bowel contents at the bottom of the figure. Arrow demonstrates the attachment of the sac to the posterior abdominal wall peritoneum. Note the morphological similarity between the sac of the peritoneal encapsulation (bottom) and normal abdominal peritoneum (top).
A large volume of ascites was also noted. The sac appeared morphologically similar to peritoneum. It enclosed the entirety of the small bowel from a point approximately 5 cm distal to the duodeno-jujenal flexure to the mid-transverse colon. The sac also encased the corresponding vascular supply, including the superior mesenteric artery (SMA) and its major branches. There was an orifice in the membrane close to the origin of the SMA to allow for entry of that vessel, at which point the membrane was found to be generating a band-like effect on the transverse colon (Fig. 3).
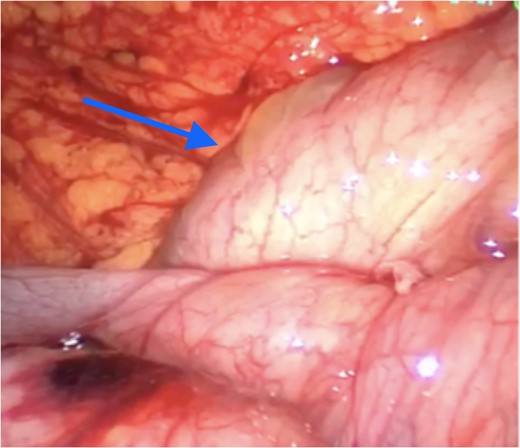
Apex of cyst (arrow) compressing transverse colon inferiorly with greater omentum visible superiorly.
After careful assessment, the decision was made to open the abdomen to allow for better visualization and complete resection of the sac. The membrane was dissected along its origin to the right iliac fossa. Numerous adhesions were noted inside the sac. The lateral boundary of the sac on the right side was noted to contain a vessel originating from the SMA trunk. This was followed down to the appendix and terminal ileum (TI). Its terminal branches were noted to end at the appendix. This vessel was clamped and ongoing strong pulsations were noted at the TI. The artery was subsequently divided and the sac released off the transverse colon. An appendicectomy was also performed. Specimens from the sac were sent for histopathological examination and the ascitic fluid was sent for cytology.
The post-operative period was uncomplicated and the patient was discharged on day 5. He remained symptomatically well at 1-month follow up.
Histopathological examination of the resected sac showed fibro-fatty tissue, with significant oedema and haemorrhage. Cytology of the intra-abdominal fluid demonstrated the presence of mesothelial cells and lymphocytes.
DISCUSSION
To our knowledge, no greater than 50 case reports of true CPE have been reported in the literature. Due to this paucity of literature and rarity of the phenomenon, there still exists conflicting understandings on the classification and aetiology of this condition and its’ relation to other bowel encapsulating diseases. Rather confusingly, CPE has often been used as a broad term to describe a number of clinical conditions with differing aetiologies. Unfortunately, the terms ‘abdominal cocoon’ and ‘encapsulating peritoneal sclerosis’ have also been wrongly used to describe CPE, which is a completely separate phenomenon with a different aetiology.
Based on the current literature, true CPE is caused by an abnormal return of the cranial mid-gut into the abdominal cavity following physiological herniation at week 12 of foetal development. As a result of this abnormal migration of bowel, the covering layer of yolk sac is made to persist over the bowel, instead of being confined to the umbilical pedicle as is normal. This additional, aberrant peritoneal layer which covers the small bowel is continuous with the transverse mesocolon superiorly and the parietal peritoneum inferiorly [2, 5]. In the case presented here, CPE was found to be an isolated pathology, however other cases have reported CPE occurring in the context of other congenital abnormalities, such as incomplete situs inversus [6].
In contrast, ‘Abdominal Cocoon’ was first described by Foo et al. [7] to describe a condition characterized by the presence of an accessory fibrous peritoneal sac that develops secondarily to chronic inflammation in the peritoneal cavity. This report suggested that perhaps the fibrous encasing was caused by retrograde menstruation in young women [7]. Since that time other reports have described a similar fibrous encapsulation being directly caused by certain specific aetiological factors, including tuberculosis, chronic ambulatory peritoneal dialysis, systemic lupus erythematosus and chronic use of certain beta-receptor blocking agents [8, 9]. In these cases where a clear aetiological factor has been present, the term encapsulating peritoneal sclerosis (EPS) has been used. This has led to some confusion in the literature in regards to appropriate nomenclature. When there is no known causative factor causing the development of a fibrous capsule, the disease is termed abdominal cocoon, with sub-clinical peritonitis of unknown cause postulated to be the aetiological basis [4, 8, 9]. When a clear aetiological factor is present, the term EPS may be used. Most importantly, these conditions are completely different from the much rarer CPE, which has aetiology based in abnormal embryonic gastrointestinal development. Reflecting these differing aetiologies, these conditions also differ markedly in appearance and histopathology. Abdominal cocoon and EPS are characterized by a thick, fibrous capsule, demonstrating the inflammatory basis of these conditions. In contrast, CPE is characterized by an additional membrane which is almost identical to peritoneum.
CONFLICT OF INTEREST STATEMENT
None declared.



