-
PDF
- Split View
-
Views
-
Cite
Cite
Sara Catarino Santos, Ana Rita Loureiro, Rosa Simão, Jorge Pereira, Luís Filipe Pinheiro, Carlos Casimiro, Wilkie’s syndrome: a case report of favourable minimally invasive surgery, Journal of Surgical Case Reports, Volume 2018, Issue 2, February 2018, rjy027, https://doi.org/10.1093/jscr/rjy027
Close - Share Icon Share
Abstract
Wilkie’s syndrome, or superior mesenteric artery syndrome (SMAS), is a rare clinical entity caused by compression of the third portion of the duodenum between the abdominal aorta and superior mesenteric artery, leading to duodenal obstruction and severe malnutrition. The authors report a case of a female patient with years of chronic intestinal obstruction with abdominal pain, vomits and weight loss. Contrast intestinal series showed dilation of stomach and duodenum. Abdominal computed tomography study revealed findings compatible with SMAS. After initial nutritional support, she was successfully treated by laparoscopic duodenojejunostomy. Surgical treatment of SMAS may be necessary in most cases with chronic symptoms or conservative treatment failure. A minimally invasive approach can be considered a safe surgical option with favourable outcomes. Clinical details, diagnostic studies and treatment are discussed.
INTRODUCTION
Wilkie’s syndrome, or superior mesenteric artery syndrome (SMAS), is a rare clinical condition characterized by partial or complete duodenal obstruction [1, 2]. It is caused by a narrowed aortomesenteric angle that leads to compression of the third portion of duodenum between the abdominal aorta (AA) and superior mesenteric artery (SMA) [2]. The syndrome was first reported in 1861 by Von Rokitansky and was studied afterwards by Wilkie who published the largest series of cases in 1927 [2–4].
The main symptoms of this condition are postprandial abdominal pain, early satiety, vomits, weight loss and malnutrition [3–5]. Diagnosis of SMAS is challenging but may be suspected based on clinical presentation and then supported by imaging studies [1, 3, 6]. SMAS may be treated medically and/or surgically [4]. Conservative management consists mainly of nutritional support [1, 6]. Surgical options are indicated for patients with chronic or refractory symptoms [3, 7]. A minimally invasive approach can be considered a good approach with favourable outcomes [3, 8].
CASE REPORT
A 39-year-old Caucasian female patient presented to a General surgery outpatient clinic with complaints of epigastric pain, nausea, vomiting and weight loss for more than 5 years. The pain was worse after eating and with supine position. Vomiting was usually initiated 3–4 h after meals and consisted of undigested food. She had no significant prior medical history. On examination, the patient was extremely emaciated, with normal vital signs but with a distended abdomen and fullness over the epigastrium. She had already done an upper endoscopy that only showed gastric stasis. Abdominal ultrasound revealed a reduced angle between the AA and SMA (Fig. 1). An upper GI contrast study was requested, which revealed a distended stomach with delayed gastric emptying and lagging of contrast at the third portion of the duodenum (Fig. 2). Abdominal computed tomography (CT) scan was then performed. It demonstrated a severe distension of the stomach and proximal portions of the duodenum with constriction of the third part of the duodenum between the AA and SMA, with a reduced angle (11°) and shortened distance (4–5 mm) between these two arteries (Figs 3 and 4). These findings were suggestive of an aortomesenteric clamp. Hence, based on known findings, the diagnosis of Wilkie’s syndrome was established.
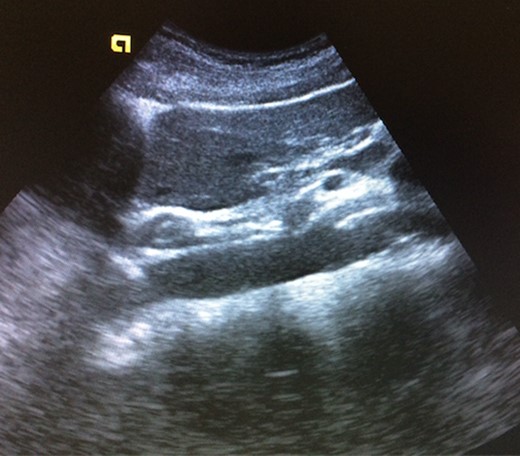
Abdominal ultrasound—reduced angle between abdominal aorta and superior mesenteric artery.
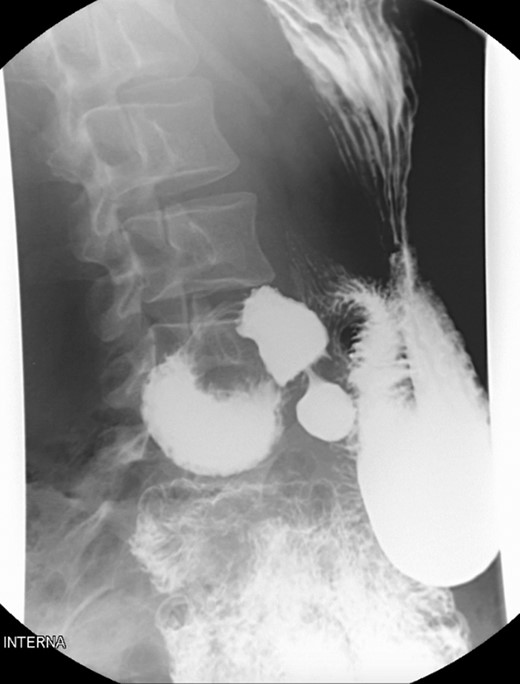
Contrasted intestinal series—distended stomach with delayed gastric emptying and reduced passage of contrast at the third portion of duodenum.
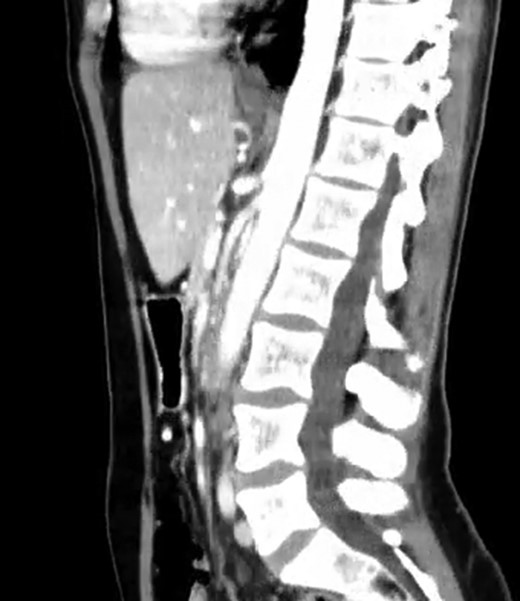
CT scan—sagittal CT image of reduced angle between AAA and SMA.
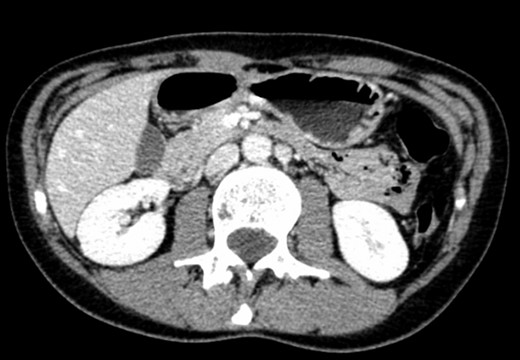
CT scan—axial CT image of obstruction of the third portion of duodenum between AAA and SMA.
Initially, conservative management was adopted with some improvement of patient’s nutrition condition. However, as symptoms persisted, surgical treatment was recommended. Our surgical approach was a laparoscopic duodenojejunostomy with a latero-lateral stapled anastomosis between jejunum (30 cm from Treitz’s angle) and the second portion of the duodenum (Figs 5–9). The patient recovered with no pain but with a delay in diet acceptance. An upper GI contrast study was performed on the fifth post-operative day revealing a distended stomach with gastric emptying delay, but with unobstructed anastomosis, therefore without stenosis or leaks (Fig. 10). She was discharged home after 8 days with liquid diet and digestive transit restored. After 3 months of follow-up, the patient gained some weight and remained asymptomatic.
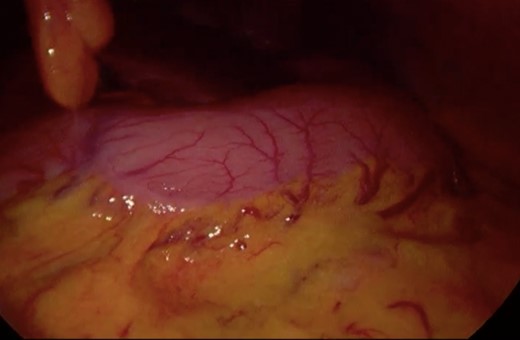
Surgery—initial laparoscopic view with distended stomach and proximal portions of duodenum.
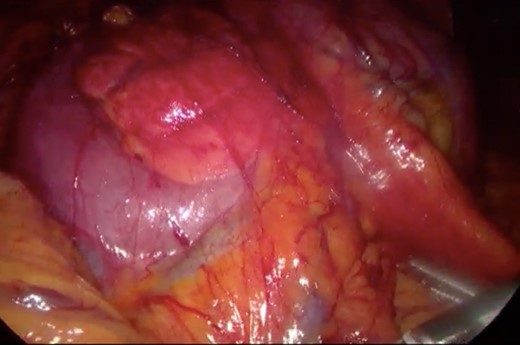
Surgery—laparoscopic visualization of the second and third portion of duodenum.
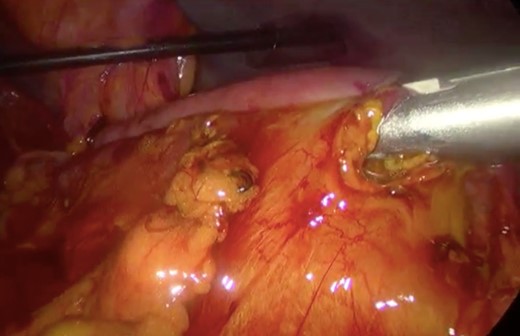
Surgery—laparoscopic stapled anastomosis between jejunum and second portion of duodenum.
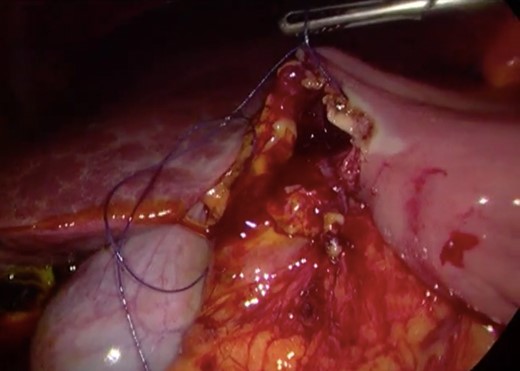
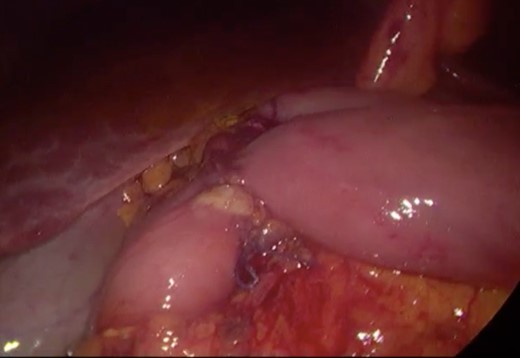
Surgery—final laparoscopic surgical view of laparoscopic duodenojejunostomy.
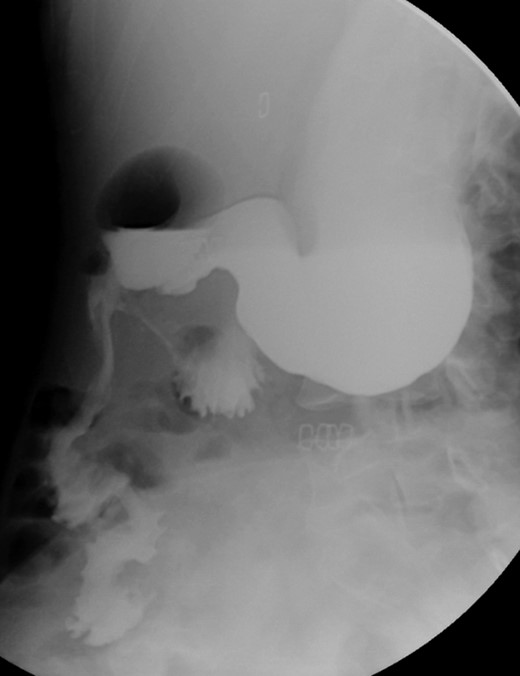
Post-operative contrasted intestinal series—delayed gastric emptying with without stenosis or leaks of the anastomosis.
DISCUSSION
SMAS, also known as Wilkie’s syndrome, mesenteric duodenal compression syndrome, chronic duodenal ileus or cast syndrome [2, 8], is a rare entity defined as a compression of the third portion of the duodenum between the SMA and AA leading to partial or complete duodenum obstruction [5, 6, 9]. The normal aortomesenteric angle and distance are 25–60° and 10–28 mm, respectively [6]. However, in SMAS this angle is narrowed to <25° and the distance is shortened to < 8 mm [7, 10]. Several factors are associated with SMAS, mainly marked weight loss as a consequence of other diseases (cancer, bariatric surgery, chronic infections, severe burns) but may also be congenital such as shorten Treitz’s ligament or abnormal origin of the SMA, or associated with surgical interventions that distorts the anatomy as scoliosis correction or esophagectomy [3, 4, 6].
SMAS has an estimated prevalence in the general population that varies between 0.013 and 0.3% [4, 6], and it most commonly affects females between 10 and 40 years of age [1, 10]. Its manifestation is complex, including postprandial epigastric pain, nausea, vomiting, early satiety, weight loss and malnutrition [3–5]. SMAS may present as an acute obstruction or it may have an insidious onset with chronic symptoms [1, 6, 9]. The diagnosis requires a high degree of suspicion [3, 8]. The low index of suspicious can lead to delays in diagnosis resulting in a chronic course of symptoms [1, 6]. Several imaging studies such as ultrasonography, CT scan, contrast digestive series and endoscopy may be necessary to confirm the diagnosis [2, 3, 8]. CT scan and magnetic resonance angiography enable visualization of the vascular compression of the duodenum and precise measurement of aortomesenteric angle and distance [1, 6].
Current therapy of SMAS consists of medical and/or surgical treatment [2, 4]. Conservative therapy includes nutritional support, left-lateral decubitus positioning, prokinetic and anti-reflux medications and fluid resuscitation [1, 6]. It is conviction of most of the authors that a trial of medical treatment should be attempted before any surgical intervention [3]. However, there is neither clear time limit nor the long-term outcome of conservative therapy [1]. Failure of conservative medical therapy or recurrent episodes of the disease are indications for surgical treatment [2, 3, 7]. Surgical options include duodenojejunostomy, gastrojejunostomy and Strong’s procedure (mobilization of the duodenum by division of the Treitz’s ligament) [1, 8]. Strong’s procedure is mostly indicated in infants, but it has a high failure rate [8, 9]. Gastrojejunostomy also presents a high risk of failure because it does not relieve the proximal duodenal obstruction and there is increased risk of peptic stomal ulceration [1, 3, 8]. Duodenojejunostomy was first introduced by Starley in 1910, and it is the current preferred treatment of SMAS with a success rate that reaches 90% [3, 8]. All these procedures can be done by a minimally invasive approach although experience is limited [8]. In 1998, Gersin and Heniford first described a laparoscopic duodenojejunostomy and this procedure spread as a viable surgical alternative, without the risks and morbidity associated with an open surgery [3]. The present case report emphasizes that a minimally invasive surgery may be a relatively easy approach, safe and effective in the definitive treatment of SMAS.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
REFERENCES
- surgical procedures, minimally invasive
- abdominal pain
- weight reduction
- duodenal obstruction
- abdominal aorta
- malnutrition
- intestinal obstruction
- intestines
- laparoscopy
- superior mesenteric artery
- nutritional support
- gastric dilatation
- superior mesenteric artery syndrome
- surgical procedures, operative
- diagnosis
- duodenum
- duodenojejunostomy
- abdominal ct
- compression
- conservative treatment



