-
PDF
- Split View
-
Views
-
Cite
Cite
Natesh Yepuri, Napat Pruekprasert, Nisha Ramani, Alexandra France, James Osei Sarpong, Ajay Jain, Robert N Cooney, Osteoclast-like giant cell tumor of the pancreas—an unusual presentation in a patient with large mantle cell lymphoma, Journal of Surgical Case Reports, Volume 2018, Issue 12, December 2018, rjy341, https://doi.org/10.1093/jscr/rjy341
Close - Share Icon Share
Abstract
Osteoclast-like giant cell tumor of the pancreas is very rare. We report a 78-year-old male who was previously treated for large mantle cell lymphoma, was found to have an increased uptake in a peri-pancreatic node from his restaging PET scan. Endoscopic ultrasound-directed fine-needle aspiration of the mass and lymph node revealed an undifferentiated carcinoma with osteoclast-like giant cells. Osteoclast-like giant cell tumors of the pancreas are frequently found to be unresectable at diagnosis due to their large size (>5 cm). In our patient, due to its small size (<3 cm) sub-total pancreatectomy was performed. Three years from the surgery, the patient is doing well without recurrence. This case report intends to increase provider awareness that in the setting of new pancreatic lesions in a patient with previous history of lymphoma, a high index of suspicion for a primary pancreatic lesion should be included in the differential diagnosis.
INTRODUCTION
Undifferentiated carcinoma of the pancreas with osteoclast‑like giant cells (UC-OGC) is an exceedingly rare, non‑endocrine tumor that accounts for less than 1% of all pancreatic malignancies [1]. UC-OGC resembles giant cell tumor of the bone containing benign appearing osteoclast-like multinucleated cells. Microscopically the presence of bland giant cells (GC) and mononuclear cells is a distinct cytomorphological feature of this rare tumor [2].
Non-Hodgkin lymphomas (NHL) involving the gastrointestinal tract are seen in 5–9% [3], while secondary pancreatic involvement is noted in 30–40% of these cases. Secondary pancreatic tumors are uncommon and account for 2–5% of pancreatic cancer. Mantle cell lymphoma (MCL) is a form of NHL that originates from mantle zone of lymph nodes. MCL is aggressive and can involve any region of the gastrointestinal tract including stomach, duodenum, jejunum, ileum, colon and rectum [3]. However, the involvement of pancreas has not been reported. Primary pancreatic lymphoma (PPL) should be differentiated from secondary pancreatic lymphoma (SPL), which may occur in up to one-third of the patients with NHL.
In the present case, the presence of a previous history of malignancy and a new pancreatic lesion confines the differential diagnosis to advanced secondary pancreatic lymphoma, which is the most common secondary pancreatic tumor, and locally advanced/metastatic pancreatic adenocarcinoma. Their clinical manifestations and radiological findings must be differentiated from other pancreatic lesions, since prognosis, treatment and survival differ from the rest.
CASE REPORT
A 78-year-old male who was previously treated for large MCL was referred to our institute after his restaging PET-CT scan showed an increased uptake in a peripancreatic node. His past medical history included prostate cancer treated with prostatectomy. He complained of fatigue, night sweats, weight loss, and abdominal pain. A CT scan revealed a centrally located mass in the head of the pancreas (Fig. 1). Endoscopic Ultrasound (EUS) revealed a 2.1 cm mass at the junction of the pancreatic body and head and peripancreatic lymph node 9.4 mm. FNA of the pancreatic mass showed scattered pleomorphic malignant cells with hyper-chromatic nuclei, prominent nucleoli and scant to moderate amount of cytoplasm. Multinucleated giant cell forms were also present (Fig. 2).
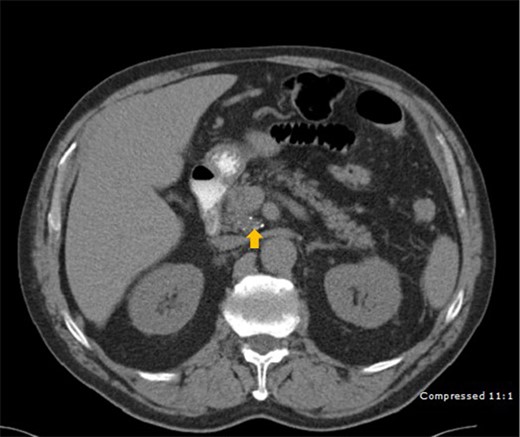
CT scan depicting a 2.5 cm mass (arrow) with calcification in the head of pancreas.
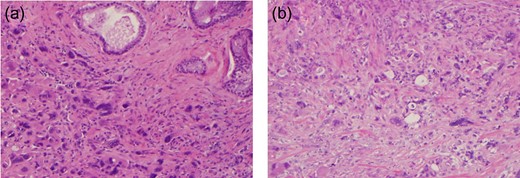
Post-operative histopathological features—(a) atypical giant cells with marked nuclear pleomorphism. (b) Desmoplastic stroma with markedly atypical giant cells with marked nuclear pleomorphism.
The patient underwent a sub-total pancreatectomy with splenectomy. Surgical pathology showed marked atypical giant cells with marked nuclear pleomorphism, multi-nucleation and increased mitotic figures consistent with UC-OGC. His post-operative course was complicated by diarrhea and hyperglycemia secondary to pancreatectomy. At present, 3 years after the surgery, the patient is doing well without recurrence.
DISCUSSION
UC-OGC and Pleomorphic giant cell carcinoma (PGCT) represent a morphologic spectrum with pure UC-OGC at one end and PGCT at the other [4]. Distinction of true UC-OGC of the pancreas from PGCT has not always been clearly documented and overlapping descriptions of these neoplasms exists in the pathology literature. In contrast to the established origin of undifferentiated carcinoma of the pancreas, there have been controversies regarding the origin of osteoclastic giant cells (OGC) [5]. GC tumors present in the sixth or seventh decade of life with a nearly equal predilection for males or females. Unlike our patient, these tumors most commonly involve the body or the tail of the pancreas rather than the head. Most of the reported cases showed masses larger than 3 cm, and more than 80% of the tumors were larger than 5 cm which reflects the rapid growth of the tumor. However, they infrequently present as small neoplasms, as in our patient. Pancreatic lymphoma (PL) affects patients in the fifth and sixth decade of life and has a slight male predominance. Ultrasound, CT and MRI as techniques that help differentiate PL from adenocarcinoma [6]. However, the definitive diagnosis is only established by the histopathological study.
On imaging UC-OGC commonly present as large cystic neoplasms with hemorrhage and necrosis [7]. Further these lesions tend to mimic more typical pancreatic adenocarcinomas except for the fact that they tend to be larger, with sizes of the primary tumor of (5–6 cm) or greater. Endoscopic ultra sonography (EUS) to some extent can help to differentiate GC tumor from typical pancreatic adenocarcinoma as they appear as heterogeneous lesions with well demarcated hyperechoic and hypoechoic areas closely opposed within the same lesion. While vascular invasion is commonly seen in adenocarcinoma, it is an uncommon finding in UC-OGC. Hence, preoperative imaging diagnosis of the tumor is often difficult.
Metastases are less likely in osteoclast-like giant cell tumor and when they do occur, it is through lymphatics or direct peritoneal extension. Accordingly, early diagnosis and complete surgical resection represent the best chance to cure this fatal tumor. The present patient is alive 3 years and disease free after the operation, GC tumors of pancreas are commonly reported to have a poor outcomes. A meta‑analysis by Kobayashi et al. compared survival of patients following surgical resection long‑term survivors (>2 years) with short‑term survivors (who succumbed <1 year) [8]. The characteristics of the short‑term survivors reported were—older age, males, positive lymph node metastasis, and concomitant components of ductal adenocarcinoma, as well as pleomorphic giant cell carcinoma. The differential diagnosis of pancreatic GC tumors includes cystic lesions like pancreatic cystadenomas, cystadenocarcinomas, serous and mucinous cystic tumors, pancreatic pseudocysts and also solid pancreatic tumors like ductal pancreatic carcinomas or neuroendocrine tumors.
The key distinguishing features between PPL and SPL [9] are summarized in Table 1. The patient in this case report did not meet any one of the criteria for PPL or SPL, except for the presence of peri-pancreatic lymph node. In our patient, the characteristic morphology of the tumor, as well as the immunophenotype support the diagnosis of a UC-OGCs.
| Primary pancreatic lymphoma—are rare entities, representing approximately 0.5% of pancreatic neoplasms and less than 2% of lymphomas. The majority of PPLs are diffuse large-cell lymphomas of intermediate to high grade and more often in men presenting in fifth to sixth decade of life. Classically, the diagnosis of PPL is established when the following criteria are met: mass that predominantly involving pancreas, involvement of peripancreatic lymph nodes, normal hemogram and absence of palpable adenopathies of mediastinal involvement and hepato-splenic metastases. Clinical symptoms are generally nonspecific; abdominal pain, mass and weight loss are the most common symptoms. Although the head of the pancreas is the most common location (>80%) of pancreatic lymphomas, jaundice is not the predominant symptom. | Secondary pancreatic lymphoma—The tumors most commonly associated with secondary pancreatic involvement are lymphoma, renal cell and lung carcinomas. SPL is far more common than other secondary pancreatic malignancies and primary pancreatic lymphoma. The most common clinical manifestations are abdominal pain, weight loss, jaundice, acute pancreatitis, small bowel obstruction and diarrhea. Obstructive jaundice is not the predominant symptom as obstruction of the common bile duct is usually absent. The classic symptoms of non-Hodgkin lymphoma such as fever, chills and night sweats are commonly seen in secondary, but are rarely present in primary pancreatic lymphoma. Key imaging findings highly suggestive of secondary pancreatic lymphoma are the absence of vascular invasion, bile and pancreatic duct obstruction, and the presence of lymphadenopathy below the level of the left renal vein. |
| Primary pancreatic lymphoma—are rare entities, representing approximately 0.5% of pancreatic neoplasms and less than 2% of lymphomas. The majority of PPLs are diffuse large-cell lymphomas of intermediate to high grade and more often in men presenting in fifth to sixth decade of life. Classically, the diagnosis of PPL is established when the following criteria are met: mass that predominantly involving pancreas, involvement of peripancreatic lymph nodes, normal hemogram and absence of palpable adenopathies of mediastinal involvement and hepato-splenic metastases. Clinical symptoms are generally nonspecific; abdominal pain, mass and weight loss are the most common symptoms. Although the head of the pancreas is the most common location (>80%) of pancreatic lymphomas, jaundice is not the predominant symptom. | Secondary pancreatic lymphoma—The tumors most commonly associated with secondary pancreatic involvement are lymphoma, renal cell and lung carcinomas. SPL is far more common than other secondary pancreatic malignancies and primary pancreatic lymphoma. The most common clinical manifestations are abdominal pain, weight loss, jaundice, acute pancreatitis, small bowel obstruction and diarrhea. Obstructive jaundice is not the predominant symptom as obstruction of the common bile duct is usually absent. The classic symptoms of non-Hodgkin lymphoma such as fever, chills and night sweats are commonly seen in secondary, but are rarely present in primary pancreatic lymphoma. Key imaging findings highly suggestive of secondary pancreatic lymphoma are the absence of vascular invasion, bile and pancreatic duct obstruction, and the presence of lymphadenopathy below the level of the left renal vein. |
| Primary pancreatic lymphoma—are rare entities, representing approximately 0.5% of pancreatic neoplasms and less than 2% of lymphomas. The majority of PPLs are diffuse large-cell lymphomas of intermediate to high grade and more often in men presenting in fifth to sixth decade of life. Classically, the diagnosis of PPL is established when the following criteria are met: mass that predominantly involving pancreas, involvement of peripancreatic lymph nodes, normal hemogram and absence of palpable adenopathies of mediastinal involvement and hepato-splenic metastases. Clinical symptoms are generally nonspecific; abdominal pain, mass and weight loss are the most common symptoms. Although the head of the pancreas is the most common location (>80%) of pancreatic lymphomas, jaundice is not the predominant symptom. | Secondary pancreatic lymphoma—The tumors most commonly associated with secondary pancreatic involvement are lymphoma, renal cell and lung carcinomas. SPL is far more common than other secondary pancreatic malignancies and primary pancreatic lymphoma. The most common clinical manifestations are abdominal pain, weight loss, jaundice, acute pancreatitis, small bowel obstruction and diarrhea. Obstructive jaundice is not the predominant symptom as obstruction of the common bile duct is usually absent. The classic symptoms of non-Hodgkin lymphoma such as fever, chills and night sweats are commonly seen in secondary, but are rarely present in primary pancreatic lymphoma. Key imaging findings highly suggestive of secondary pancreatic lymphoma are the absence of vascular invasion, bile and pancreatic duct obstruction, and the presence of lymphadenopathy below the level of the left renal vein. |
| Primary pancreatic lymphoma—are rare entities, representing approximately 0.5% of pancreatic neoplasms and less than 2% of lymphomas. The majority of PPLs are diffuse large-cell lymphomas of intermediate to high grade and more often in men presenting in fifth to sixth decade of life. Classically, the diagnosis of PPL is established when the following criteria are met: mass that predominantly involving pancreas, involvement of peripancreatic lymph nodes, normal hemogram and absence of palpable adenopathies of mediastinal involvement and hepato-splenic metastases. Clinical symptoms are generally nonspecific; abdominal pain, mass and weight loss are the most common symptoms. Although the head of the pancreas is the most common location (>80%) of pancreatic lymphomas, jaundice is not the predominant symptom. | Secondary pancreatic lymphoma—The tumors most commonly associated with secondary pancreatic involvement are lymphoma, renal cell and lung carcinomas. SPL is far more common than other secondary pancreatic malignancies and primary pancreatic lymphoma. The most common clinical manifestations are abdominal pain, weight loss, jaundice, acute pancreatitis, small bowel obstruction and diarrhea. Obstructive jaundice is not the predominant symptom as obstruction of the common bile duct is usually absent. The classic symptoms of non-Hodgkin lymphoma such as fever, chills and night sweats are commonly seen in secondary, but are rarely present in primary pancreatic lymphoma. Key imaging findings highly suggestive of secondary pancreatic lymphoma are the absence of vascular invasion, bile and pancreatic duct obstruction, and the presence of lymphadenopathy below the level of the left renal vein. |
CONCLUSION
Pancreatic undifferentiated carcinomas with OGCs are very rare neoplasms and can present with an atypical clinical picture. We present the first reported case of a pancreatic UC-OGC in a large MCL patient. We raise the index of suspicion of two primary tumors in patients with underlying malignant disease.
CONFLICT OF INTEREST STATEMENT
None declared.



