-
PDF
- Split View
-
Views
-
Cite
Cite
Aldo Fafaj, Federico N Aucejo, Erica Savage, Toms Augustin, A unique case of xanthogranulomatous cholecystitis complicated by multiple liver abscesses and portal vein and hepatic artery thrombosis and occlusion, Journal of Surgical Case Reports, Volume 2018, Issue 12, December 2018, rjy337, https://doi.org/10.1093/jscr/rjy337
Close - Share Icon Share
Abstract
Xanthogranulomatous cholecystitis (XGC) is difficult to diagnose preoperatively because it often mimics gallbladder cancer. We present a case of a 64-year-old Caucasian male who presented with multiple intrahepatic abscesses, left portal vein and segmental right hepatic arterial thrombosis suspicious for extrahepatic cholangiocarcinoma who ultimately underwent an extended left hepatectomy and was noted to have XGC on final pathology. This case presents a new challenge in diagnosing XGC prior to final pathology results given the unique left portal vein, and later, right anterior portal vein thrombosis and occlusion. XGC should be in the differential diagnosis when diffuse gallbladder wall thickening is associated with involvement of biliary and vascular structures. While diagnosing these cases can be challenging, increased awareness of varied involvement of the liver and hilar structures associated with this diseases process may aid in the selection of the most appropriate surgical techniques.
INTRODUCTION
Xanthogranulomatous cholecystitis (XGC) is a rare variant of chronic cholecystitis characterized by lipid-laden macrophages causing xanthogranulomas, foam cells and severe fibrosis [1, 2]. Although it is found in 1–6% of cholecystectomies worldwide, the etiology of the disease is not well-known [3]. In the most severe cases, inflammation and fibrosis may extend into the adjacent tissues mimicking advanced gallbladder cancer which makes diagnosis and management of this disease challenging [4]. We present a unique case of XGC which was characterized by inflammatory involvement of surrounding organs causing portal vein involvement and occlusion, occlusion of secondary biliary radicles and multiple liver abscess concerning for extrahepatic cholangiocarcinoma versus gallbladder cancer. While there are several reports of XGC appearing indistinguishable from advanced gallbladder cancer the authors were unable to find any reported cases of XGC leading to portal vein occlusion.
CASE REPORT
A 64-year-old Caucasian male who was treated over the course of 7 months for multiple intrahepatic abscesses, left portal vein and segmental hepatic arterial thrombosis ultimately underwent an extended left hepatectomy and was found to have XGC. The patient had a medical history significant for non-insulin dependent diabetes, chronic renal failure, chronic hepatitis C and coronary artery disease. He initially presented to the hospital seven months prior to his eventual surgery with diabetic ketoacidosis and at the time was found to have hypodense areas in the lateral and medial segments of the left lobe with extension to the hilum concerning for evolving phlegmon of the liver. Subsequent ultrasound of right upper quadrant revealed acute left portal vein thrombus (Fig. 1). The patient was started on heparin infusion and transitioned to coumadin. He was subsequently readmitted with sepsis and underwent an attempt at image-guided drainage of the liver abscess (Fig. 2) where no purulent fluid could be aspirated. A solid lesion was noted which was biopsied with final pathology noting organizing hematoma with acute and chronic inflammation.
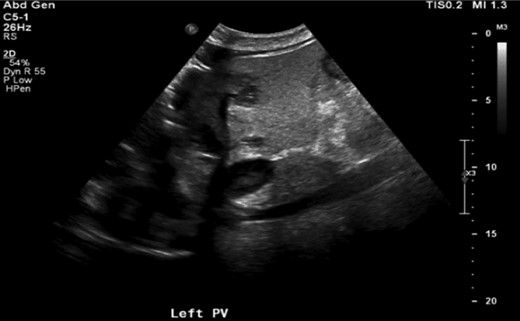
Right upper quadrant ultrasound showing left portal vein thrombosis (arrow).
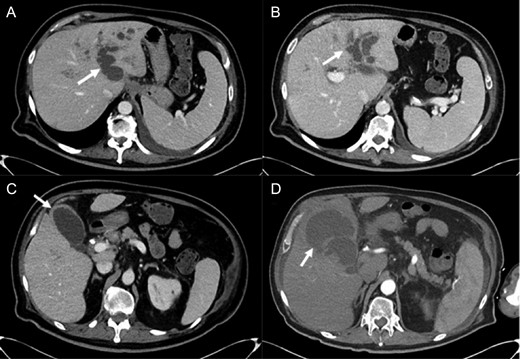
Abdominal imaging findings: axial computed tomography (CT) scan showing the intrahepatic dilation of the left sided biliary system (arrow) from cranial (A) to caudal (B), diffusely thickened gallbladder wall (C, arrow) and left intrahepatic abscess (D, arrow).
Repeat CT imaging revealed continued thrombosis of the left portal vein and anterior branches of the right portal vein and right hepatic artery (Fig. 3). Due to persistent left intrahepatic abscess and concern for an underlying obstructive process in the left biliary system, the patient underwent an endoscopic retrograde cholangiopancreatography (ERCP) which revealed choledocholithiasis which was removed followed by sphincterotomy and stent placement. He subsequently underwent a spyglass™ procedure which noted a left biliary tract occlusion secondary to a mass which was biopsied. Pathology, however, was noted to be benign with debris and bile crystalline material and rare fragments of fibrous tissue with biliary-type epithelium.
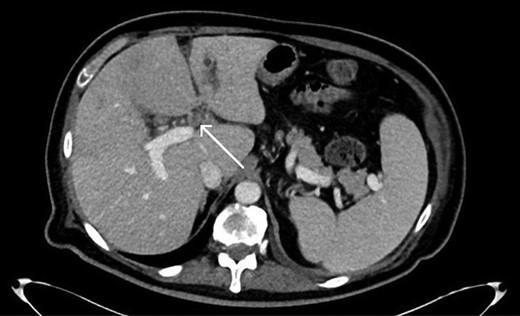
Axial CT scan showing thrombosis of the left portal vein (arrow).
After evaluation and review of his complicated hospital course with no clear diagnosis but high suspicion for cholangiocarcinoma, a decision was made to undergo a diagnostic laparoscopy with peritoneal washings to rule out carcinomatosis given ascites. The laparoscopy noted inflammation in segments 4 and 5 of the liver, with the gallbladder not visualized since it was covered by omentum. Several biopsies which were obtained from both the right and left liver segments including washings were negative for malignant cells. Thus, the decision was made to proceed with hepatectomy.
Preoperatively patient reported mild right upper quadrant pain, nausea and diarrhea. He denied any fevers, jaundice or weight loss. On physical exam, his vitals were stable, the abdomen was soft and non-tender to palpation. Labs showed white blood cell (WBC) of 3.84 k/uL, hemoglobin 9.4 g/dL, aspartate aminotransferase (AST) 56 U/L, alanine aminotransferase (ALT) 63 U/L, alkaline phosphatase 261 U/L and total bilirubin 1.0 mg/dL. Tumor marker noted cancer antigen CA 19-19 at 53 U/mL (normal limit: <36 U/mL) and carcinoembryonic antigen (CEA) at 1.7 ng/mL (normal limit <2.9 ng/mL).
Intraoperatively, the surface of the liver was noted to be quite inflamed. There were dense pericholecystic adhesions, and the gallbladder was diffusely thickened. A hard mass with surrounding inflammatory and fibrotic changes was encountered in segment IV. After mobilizing the liver and controlling all hilar structures, in addition to Doppler confirmation of right hepatic artery flow, an extended left hepatectomy was performed. The right hepatic artery was carefully dissected off the mass, and the left liver including segments 5 and 8 as well as the caudate lobe was resected (Fig. 4). We were able to preserve the main bile duct. Pathology subsequently revealed XGC extending into the hepatic parenchyma (Fig. 5). After an uneventful post-operative course patient was discharged to extended care facility in stable condition 8 days after his surgery.
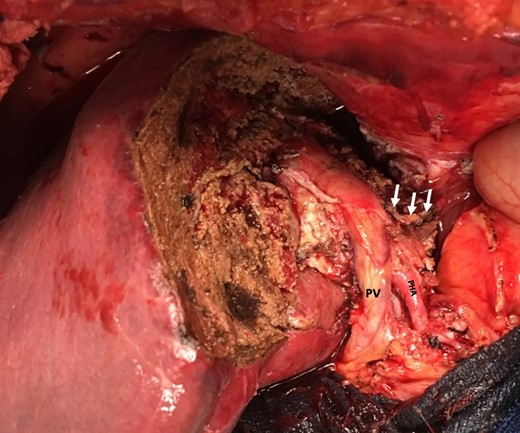
Remnant liver after left extended hepatectomy with preserved hilar structures (portal vein (PV), proper hepatic artery (PHA) and staple line after resection of left portal vein (arrows)).
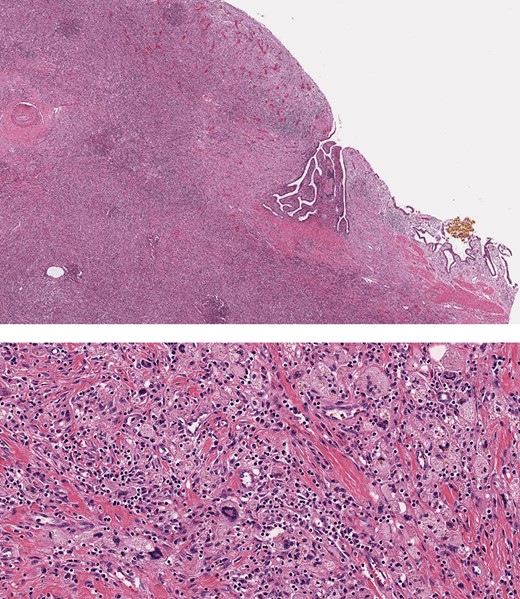
Histopathology, showing transmural inflammatory process in the gallbladder with mucosal ulceration (top) and the mass lesion composed of sheets of foamy histiocytes admixed with plasma cells, lymphocytes, collagen fibers and scattered giant cells (bottom).
DISCUSSION
XGC is a poorly understood and rare gallbladder disease that can mimic gallbladder cancer. There are several theories about pathophysiology, but the most widely cited one hypothesizes that increased intraluminal pressure in the gallbladder causes extravasation of bile into the gallbladder wall which is then taken up by activated histiocytes and fibroblasts causing a granulomatous reaction followed by cellular immune response and fibrosis [4, 5].
Establishing the correct diagnosis of XGC remains challenging, especially when the inflammatory process involves the surrounding tissues, the diagnoses is frequently delayed until the final pathology results [4]. Waskin et al. [6] studied multi detector CT as an imaging modality to detect XGC and showed a moderate sensitivity but poor specificity for differentiation of XGC from gallbladder cancer. Additionally, a scoring system proposed by Rajaguru et al.[7] to aid in diagnosing XGC preoperatively, does not account for any vascular involvement. Thus, the preoperative diagnosis of XGC remains challenging.
This case highlights the unique variation of the pathologic and imaging spectrum of XGC with biliary, vascular and hepatic parenchymal involvement reminiscent of malignant disease presentation. Additionally, the left portal vein and later right anterior portal vein thrombosis requiring anticoagulation, which, at the time of this writing, has not been described in the literature. XGC should be considered in the differential diagnosis of liver and bile duct mass associated with gallbladder wall thickening and cholelithiasis [8], understanding that the coexistence of gallbladder cancer and XGC has been observed in 0–20% of patients [9]. Vascular involvement, as was noted in our patient, should not exclude XCG from the differential. Ultimately, the management decision should be a shared decision between the patient and the surgeon after taking into consideration all preoperative evaluations, comorbidities and symptoms in order to ensure an optimal surgical outcome.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- cholecystitis
- arterial thrombosis
- cholangiocarcinoma
- gallbladder cancer
- hepatic abscess
- abscess
- differential diagnosis
- portal vein
- surgical procedures, operative
- european continental ancestry group
- liver
- pathology
- portal vein thrombosis
- hepatic artery thrombosis
- hepatectomy, total left lobectomy
- left hepatic portal vein
- gallbladder wall thickening



