-
PDF
- Split View
-
Views
-
Cite
Cite
Srinivas Bojanapu, Vivek Mangla, Siddharth Mehrotra, Shailendra Lalwani, Naimish Mehta, Samiran Nundy, Brunner’s gland hyperplasia: an unusual duodenal submucosal lesion seen in four patients, Journal of Surgical Case Reports, Volume 2018, Issue 11, November 2018, rjy305, https://doi.org/10.1093/jscr/rjy305
Close - Share Icon Share
Abstract
Brunner’s gland hyperplasia is an extremely rare benign hamartomatous lesion seen in proximal duodenum. Difficulty in diagnosing the condition pre-operatively puts the surgeon in dilemma for deciding appropriate management. We retrieved details from prospectively maintained retrospective data from January 2014 to April 2018. Four patients were identified of which three were males and one was female. Symptoms ranged from 4 days to 4 years, with abdominal pain, vomiting and malena being predominant. No patients were identified with diagnosis pre-operatively. Diagnosis was made on histopathological examination of the resected specimen and none of them were having malignant features. At a median follow up of 11 months, no patient had recurrence and were symptom free. Brunner’s gland hyperplasia is a rare elusive duodenal pathology, symptomatically mimicking alarming duodenal lesions and mostly diagnosed on histopathology of specimen. Patients may harbour the lesion for long periods with little symptoms and upon treatment have good outcomes.
BACKGROUND
A Brunner’s gland adenoma is a rare benign hamartomatous lesion characterized by the proliferation of Brunner’s glands and has been variably called a Brunner’s gland adenoma, hamartoma, hyperplasia or a Brunneroma in the literature. We describe our management of four patients with such lesions who presented with clinical symptoms and underwent surgery.
METHODS
We retrieved, from a prospectively maintained database, the records of all our patients (between 2014 and 2018) who presented with symptoms related to duodenal lesions requiring surgical intervention. Four patients had Brunner’s gland hyperplasia on histopathological examination. Incidental findings of Brunner’s gland hyperplasia after a procedure performed for a different preoperative diagnosis (e.g. pancreaticoduodenectomy for proven cancer/neuroendocrine tumours) were excluded. Their postoperative complications were graded according to the Clavien Dindo scale.
RESULTS
There were three males and one female who had Brunner’s gland hyperplasia as the pathological finding in their surgical specimens following operations for duodenal lesions. Their mean age at presentation was 39.7 ± 10.14 (range: 30–52) years and their presenting features were abdominal pain in two patients, vomiting (2), and upper gastrointestinal bleeding (2). The duration of their symptoms varied between 4 days and 4 years prior to presentation. All patients underwent an upper gastrointestinal endoscopy (Figs 1 and 2) and abdominal computed tomography(Figs 3 and 4) scans as part of their evaluation. The details of patient presentation, evaluation, surgical procedure and outcomes are described in Table 1.
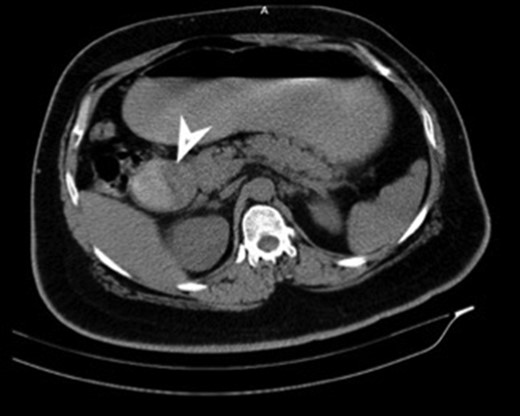
CT (transverse) image showing first and second part of duodenum filled with a mass lesion.
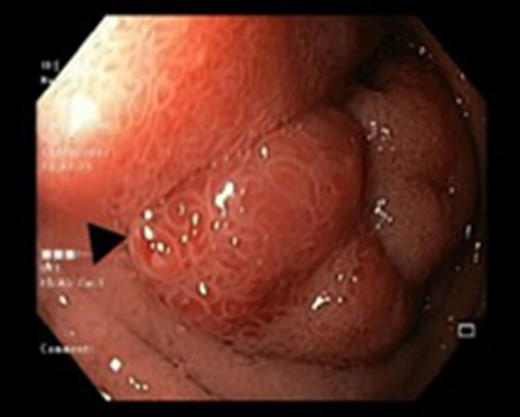
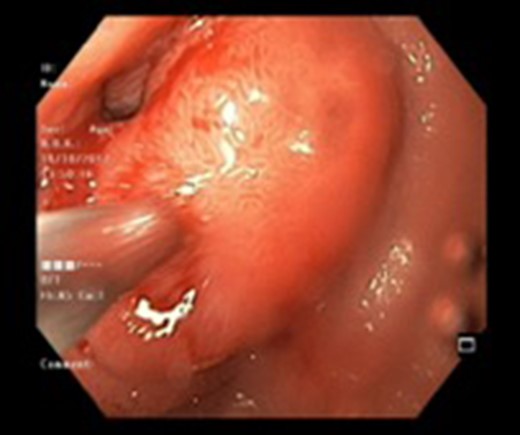
Endoscopic view of a submucosal mass in the second part of the duodenum.
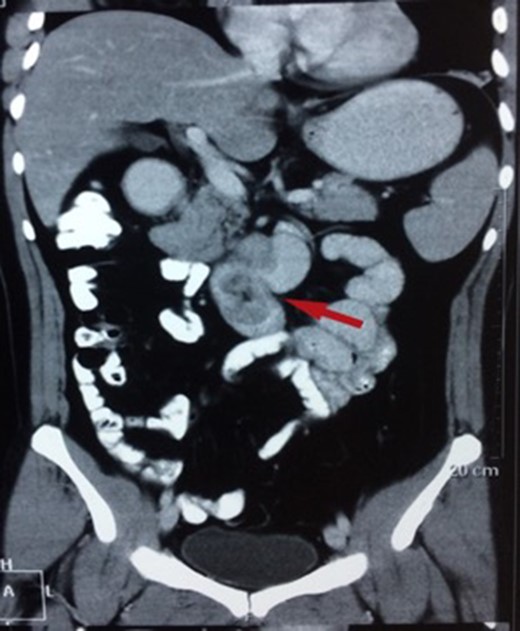
CT showing a polypoid lesion extending from the first part of the duodenum and projecting into the lumen of the second and third part.
| S. No. . | Age/Sex . | Symptom duration . | Symptoms . | Preoperative CT scan . | Endoscopic findings . | Pre-op tissue biopsy . | Operative procedure . | Clavien grade . | Post- op ICU stay . | Post-op hospital stay . |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 30/M | 3 months | Vomiting, Malena | Well defined rounded hypodense submucosal lesion in second part of duodenum (Fig. 1) | Smooth mucosal bulge likely due to submucosal lesion in D2 with difficulty in negotiating scope beyond (Figs 2 and 3) | Normal duodenal mucosal fragments with mild lympho-mono nuclear cell infiltrate in the lamina propria | Pancreaticoduodenectomy | 2 | 2 days | 6 |
| Case 2 | 44/M | 4 days | Malena, fatigue requiring blood transfusions | 60 × 45 mm2 mildly enhancing solid mass involving second and third part of duodenum with exophytic contour bulge likely polypoid lesion (Fig. 4) | A Large friable polypoidal lesion at junction of second and third part of duodenum | Non-specific chronic duodenitis with focal mild dysplasia | Transduodenal polyp excision (Fig. 5) | 2 | 1 | 5 |
| Case 3 | 33/M | 4 years | Abdominal pain, vomiting | Poorly circumscribed lesion of head of pancreas of size 5 × 4.4 cm2 compressing duodenum | Edematous folds, at D1-D2 junction | ? Papillary epithelial neoplasm of pancreas | Pancreaticoduodenectomy | 2 | 2 | 8 |
| Case 4 | 52/F | 1 year | Abdominal pain, regurgitation | 2 × 1.5 cm2 enhancing polypoidal lesion from medial wall of first part of duodenum? leiomyoma | A 2 × 2 cm2 polypoidal lesion starting from antrum extending into duodenum | ? Adenomatous polyp | Billroth II procedure | 2 | 0 | 7 |
| S. No. . | Age/Sex . | Symptom duration . | Symptoms . | Preoperative CT scan . | Endoscopic findings . | Pre-op tissue biopsy . | Operative procedure . | Clavien grade . | Post- op ICU stay . | Post-op hospital stay . |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 30/M | 3 months | Vomiting, Malena | Well defined rounded hypodense submucosal lesion in second part of duodenum (Fig. 1) | Smooth mucosal bulge likely due to submucosal lesion in D2 with difficulty in negotiating scope beyond (Figs 2 and 3) | Normal duodenal mucosal fragments with mild lympho-mono nuclear cell infiltrate in the lamina propria | Pancreaticoduodenectomy | 2 | 2 days | 6 |
| Case 2 | 44/M | 4 days | Malena, fatigue requiring blood transfusions | 60 × 45 mm2 mildly enhancing solid mass involving second and third part of duodenum with exophytic contour bulge likely polypoid lesion (Fig. 4) | A Large friable polypoidal lesion at junction of second and third part of duodenum | Non-specific chronic duodenitis with focal mild dysplasia | Transduodenal polyp excision (Fig. 5) | 2 | 1 | 5 |
| Case 3 | 33/M | 4 years | Abdominal pain, vomiting | Poorly circumscribed lesion of head of pancreas of size 5 × 4.4 cm2 compressing duodenum | Edematous folds, at D1-D2 junction | ? Papillary epithelial neoplasm of pancreas | Pancreaticoduodenectomy | 2 | 2 | 8 |
| Case 4 | 52/F | 1 year | Abdominal pain, regurgitation | 2 × 1.5 cm2 enhancing polypoidal lesion from medial wall of first part of duodenum? leiomyoma | A 2 × 2 cm2 polypoidal lesion starting from antrum extending into duodenum | ? Adenomatous polyp | Billroth II procedure | 2 | 0 | 7 |
M, Male; F, Female; cm, centimetre.
| S. No. . | Age/Sex . | Symptom duration . | Symptoms . | Preoperative CT scan . | Endoscopic findings . | Pre-op tissue biopsy . | Operative procedure . | Clavien grade . | Post- op ICU stay . | Post-op hospital stay . |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 30/M | 3 months | Vomiting, Malena | Well defined rounded hypodense submucosal lesion in second part of duodenum (Fig. 1) | Smooth mucosal bulge likely due to submucosal lesion in D2 with difficulty in negotiating scope beyond (Figs 2 and 3) | Normal duodenal mucosal fragments with mild lympho-mono nuclear cell infiltrate in the lamina propria | Pancreaticoduodenectomy | 2 | 2 days | 6 |
| Case 2 | 44/M | 4 days | Malena, fatigue requiring blood transfusions | 60 × 45 mm2 mildly enhancing solid mass involving second and third part of duodenum with exophytic contour bulge likely polypoid lesion (Fig. 4) | A Large friable polypoidal lesion at junction of second and third part of duodenum | Non-specific chronic duodenitis with focal mild dysplasia | Transduodenal polyp excision (Fig. 5) | 2 | 1 | 5 |
| Case 3 | 33/M | 4 years | Abdominal pain, vomiting | Poorly circumscribed lesion of head of pancreas of size 5 × 4.4 cm2 compressing duodenum | Edematous folds, at D1-D2 junction | ? Papillary epithelial neoplasm of pancreas | Pancreaticoduodenectomy | 2 | 2 | 8 |
| Case 4 | 52/F | 1 year | Abdominal pain, regurgitation | 2 × 1.5 cm2 enhancing polypoidal lesion from medial wall of first part of duodenum? leiomyoma | A 2 × 2 cm2 polypoidal lesion starting from antrum extending into duodenum | ? Adenomatous polyp | Billroth II procedure | 2 | 0 | 7 |
| S. No. . | Age/Sex . | Symptom duration . | Symptoms . | Preoperative CT scan . | Endoscopic findings . | Pre-op tissue biopsy . | Operative procedure . | Clavien grade . | Post- op ICU stay . | Post-op hospital stay . |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 30/M | 3 months | Vomiting, Malena | Well defined rounded hypodense submucosal lesion in second part of duodenum (Fig. 1) | Smooth mucosal bulge likely due to submucosal lesion in D2 with difficulty in negotiating scope beyond (Figs 2 and 3) | Normal duodenal mucosal fragments with mild lympho-mono nuclear cell infiltrate in the lamina propria | Pancreaticoduodenectomy | 2 | 2 days | 6 |
| Case 2 | 44/M | 4 days | Malena, fatigue requiring blood transfusions | 60 × 45 mm2 mildly enhancing solid mass involving second and third part of duodenum with exophytic contour bulge likely polypoid lesion (Fig. 4) | A Large friable polypoidal lesion at junction of second and third part of duodenum | Non-specific chronic duodenitis with focal mild dysplasia | Transduodenal polyp excision (Fig. 5) | 2 | 1 | 5 |
| Case 3 | 33/M | 4 years | Abdominal pain, vomiting | Poorly circumscribed lesion of head of pancreas of size 5 × 4.4 cm2 compressing duodenum | Edematous folds, at D1-D2 junction | ? Papillary epithelial neoplasm of pancreas | Pancreaticoduodenectomy | 2 | 2 | 8 |
| Case 4 | 52/F | 1 year | Abdominal pain, regurgitation | 2 × 1.5 cm2 enhancing polypoidal lesion from medial wall of first part of duodenum? leiomyoma | A 2 × 2 cm2 polypoidal lesion starting from antrum extending into duodenum | ? Adenomatous polyp | Billroth II procedure | 2 | 0 | 7 |
M, Male; F, Female; cm, centimetre.
Two patients underwent a pancreaticoduodenectomy, one underwent distal gastrectomy with Billroth II gastrojejunostomy, and one patient underwent transduodenal polyp excision (Fig. 5). Their mean ICU stay was 1.25 ± 0.95 (range: 0–4) days and postoperative hospital stay was 6.5 days (range: 5–8 days). Two patients had Grade I and two patients had Grade II complications. One patient required blood transfusion and other required prolonged nasogastric tube retention in view of persistent vomiting. There was no mortality. All patients are on regular follow up and after a median duration of 11 months they are all symptom free.
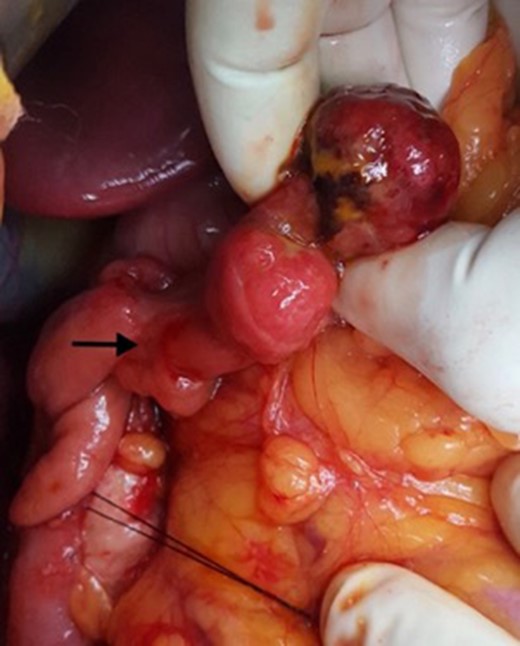
Intra-operative photograph showing the polypoid lesion in the second part of duodenum after a duodenotomy.
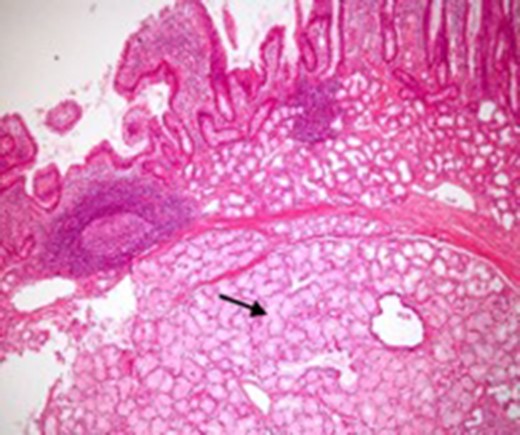
Microscopic appearance of Brunner’s gland hyperplasia. Arrow pointing at Brunner’s glands in the submucosa (stained by H & E).
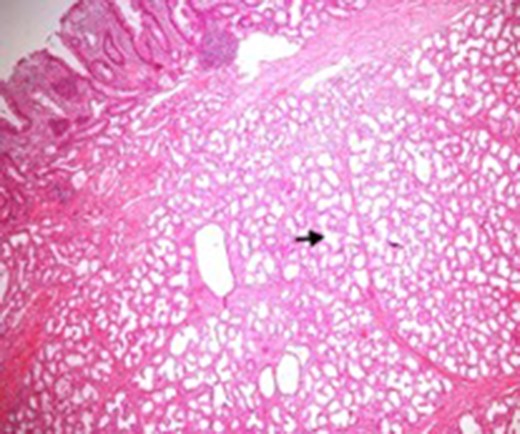
Microscopic appearance of Brunner’s gland hyperplasia. Arrow pointing at Brunner’s glands in the submucosa (stained by H & E).
DISCUSSION
Brunner’s glands are branched acinotubular glands localized in the submucosal layer of the duodenum, predominantly in its most proximal part, and there is a decrease in prevalence in the second and third parts of the duodenum. They were first described in 1688 by Johann Brunner, a Swiss anatomist. Brunner’s glands secrete an alkaline fluid that protects the epithelial lining of the duodenum from the acid chyme of the stomach [1–3].
A Brunner’s gland adenoma is a rare tumour-like lesion, mostly seen in middle age without any sex predilection. The aetiology of Brunner’s gland adenoma remains obscure. It has been postulated that an increased gastric acid secretion could stimulate these structures to undergo hyperplasia [4]. A Brunner’s gland adenoma is generally a single pedunculated polyp, with an average size of 2 cm, rarely larger than 5 cm, and usually located in the first part of the duodenum [5].
Most patients with Brunner’s gland adenoma are asymptomatic or have non-specific complaints such as nausea, bloating, or vague abdominal pain [6]. In these cases, the lesion is usually an incidental finding detected during endoscopy or imaging studies. The most common presentations in symptomatic patients are gastrointestinal bleeding and gastric outlet obstruction.
The largest series reported in the literature by Levine et al. studied the characteristics of a group of 27 patients with Brunner’s gland adenomas including those who were asymptomatic. They found that a large number of patients with tumour-related blood loss had melaena and showed evidence of chronic bleeding with ulceration, while 37% patients presented with gastric outlet obstruction. They reported that asymptomatic patients had smaller lesions (mean, 1.6 cm), and patients with obstructive and bleeding symptoms had similar-sized lesions (mean: 2.1 and 2.8 cm, respectively) [7].
These lesions are often difficult to differentiate from other submucosal lesions in this location pre-operatively from gastrointestinal stromal and neuroendocrine tumours. Large adenomas can be detected by ultrasonography and computed tomography [8]. CT is also useful to confirm the absence of extra-luminal extension of a Brunner’s gland adenoma [9]. In our series, all patients were symptomatic at presentation and were being evaluated for these, and lesions found incidentally (e.g. after pancreaticoduodenectomy specimens for cancer) were excluded.
Endoscopy can localize the lesion; however, biopsies are usually negative. Only a deep endoscopic or a surgical biopsy provides adequate tissue because the Brunner’s gland proliferations are usually covered by normal mucosa [10] (Figs 6 and 7). It is prudent to include a Brunner gland hyperplasia in a differential diagnosis while evaluating a duodenal mass, since it has varied presentation and bleeding is a common clinical sign masquerading other gastrointestinal conditions, e.g. gastrointestinal stromal tumours.
In conclusion, Brunner’s gland tumours are benign uncommon tumours and symptomatic patients usually present with vomiting, bleeding and abdominal pain all of which are of a non-specific nature. Preoperative diagnosis is unusual as they are rare and they are usually found on histopathological examination of surgically resected specimens. After surgical extirpation they are associated with good short- and long-term outcomes.
CONFLICT OF INTEREST STATEMENT
None declared.
AUTHOR’S CONTRIBUTION
Dr Srinivas Bojanapu: conception, acquisition, design, drafting, final approval agreement to be accountable. Dr Vivek Mangla: conception, design, interpretation, critical revising, final approval. Dr Siddharth Mehrotra: acquisition, revising for important intellectual content. Dr Shailendra Lalwani: acquisition, revising for important intellectual content. Dr Naimish Mehta: acquisition, revising for important intellectual content, final approval. Dr Samiran Nundy: contribution to conception, interpretation, critical revising, final approval.



