-
PDF
- Split View
-
Views
-
Cite
Cite
Eoghan Burke, Munir Saeed, Paul Anant, Babur Sami, Mohamed Salama, Ibrahim Ahmed, Large intra-abdominal desmoid tumour posing diagnostic and therapeutic challenges, a case report, Journal of Surgical Case Reports, Volume 2018, Issue 11, November 2018, rjy304, https://doi.org/10.1093/jscr/rjy304
Close - Share Icon Share
Abstract
We present the case of a 46-year-old gentleman originally from China who presented to the acute surgical assessment unit complaining of upper abdominal discomfort, dyspepsia and early satiety ongoing for the previous 6 months. On exam he had a palpable mass in the left upper quadrant. He underwent an esophagogastroduodenoscopy which was normal and later received a CT abdomen which identified a well-circumscribed soft tissue mass in the mesenteric fat and lying adjacent to the transverse colon with no obvious cleavage plane between them. Colonoscopy was then performed which was normal. After discussion at MDT he was taken for laparotomy. At laparotomy the mass was found to be adherent to major vessels, small bowel and large bowel necessitating an extended right hemicolectomy and small bowel resection. The mass itself could not be completely excised. Histology from the resected specimen confirmed desmoid tumour.
INTRODUCTION
Desmoid tumours (DTs) are benign locally aggressive mesenchymal neoplasms derived from musculoaponeurotic stromal elements [1]. The term ‘desmoid’ is itself derived from the Greek word desmos meaning band or tendon like. DTs are rare accounting for ~0.03% of all neoplasms and <3% of all soft tissue neoplasms [2]. They are most often encountered sporadically, slightly more commonly in women and tend to affect the abdominal wall and soft tissues of the extremities particularly around the shoulder and neck. Whilst they have no documented ability to metastasize they can be locally aggressive with destruction of surrounding tissues and frequently recur [3].
A yet rarer form of this rare pathology is the intra-abdominal DT. This subtype is associated with familial adenomatous polyposis syndrome (FAP) and, due to its intra-abdominal location and involvement of vital organs, proves even more difficult to treat with surgical resection being the corner stone of current management. Recurrence rates following surgical excision have been reported as high as 75%. Other treatment options include systemic therapy and radiation therapy. The natural history of DTs is highly variable with some spontaneously regressing whilst others relentlessly progress and can grow to massive proportions [4].
CASE REPORT
We report the case of Mr X, a fit and healthy 46-year-old Chinese gentleman with no previous medical or surgical history who presented to the acute surgical assessment unit complaining of a 6-month history of vague abdominal discomfort. On further questioning he revealed his pain was localized predominantly to the left upper quadrant (LUQ), was dull in nature and associated with nausea, dyspepsia and early satiety. On physical exam there was a palpable mass in the LUQ which was smooth and non tender. Laboratory investigations were all within normal limits.
Mr X underwent an esophagogastroduodenoscopy which did not identify any mass or abnormality. He later received a CT abdomen which displayed a well circumscribed 5.5 cm × 5.5 cm × 5.6 cm mass projecting from the mesenteric fat and lying adjacent to the transverse colon with no identifiable cleavage plane between them (Fig. 1). There were no pathologically enlarged lymph nodes identified. Mr X subsequently underwent colonoscopy which was normal.
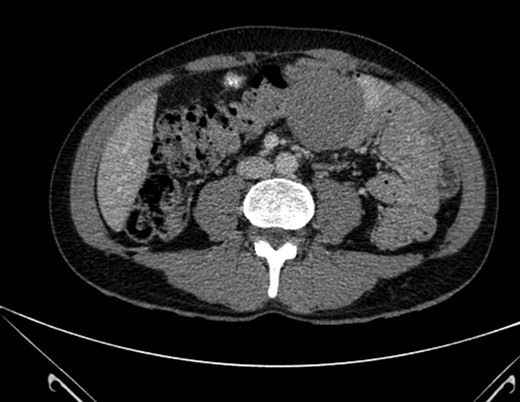
Well-circumscribed mass measuring 5.5 × 5.6 cm2 projecting from the mesenteric fat and lying adjacent to the transverse colon with no identifiable cleavage plane between them. No pathological lymphadenopathy identified.
His case was discussed at a gastrointestinal multi-disciplinary meeting with differential diagnoses of primary colorectal cancer, large gastrointestinal stromal tumour (GIST) and DT all considered. It was elected that Mr X undergo laparotomy with excision of this mesenteric mass.
On opening the abdomen the mass was found to be infiltrating surrounding structures including the small bowel and had encased itself around the superior mesenteric artery and vein. Blood supply to the small bowel was compromised and the mass was adherent to the cecum necessitating an extended right hemicolectomy, small bowel resection, jejuno-jejunal anastomosis and a ileo-colic anastomosis.
Unfortunately it was not possible to excise the mass intraoperatively and a sample was sent for histology.
Histological examination identified hypocellular spindle cells which can be seen to infiltrate between adjacent adipocytes and which stained positive for beta-catenin but stained negative for the GIST markers CD117 and DOG-1 (Fig. 2) supporting the diagnosis of DT.
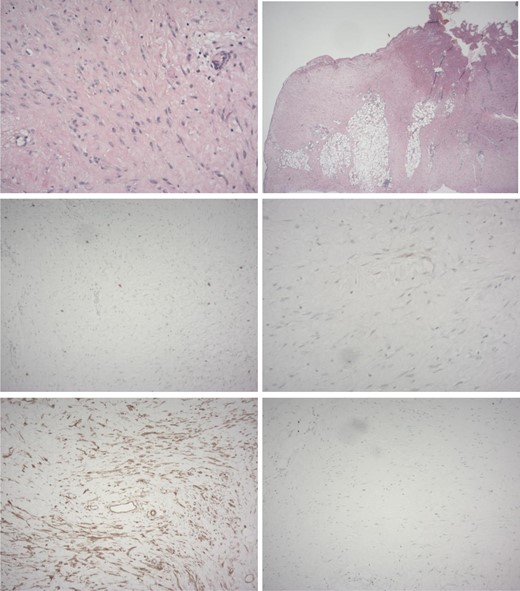
Histology of resected specimen. From top to bottom and left to right. (A) Haematoxylin and eosin staining showing spindle cells (B) spindle cells can be seen infiltrating adipose tissue. (C) Staining negative for CD117 and (D) DOG-1. (E) staining strongly positive for beta catenin. (F) Weak Ki67 staining. Supporting the diagnosis of Desmoid tumour.
His lesion is currently inoperable and systemic treatment options are being considered whilst his mass is kept under radiological surveillance.
DISCUSSION
DTs are incredibly rare with approximately only 900 new cases each year in the USA [5]. The underlying pathophysiology behind these lesions is poorly understood, however, there is growing evidence to support the involvement of the Adenomatous Polyposis Coli tumour suppressor and beta-catenin genes in both sporadic and FAP associated DTs [6].
Intra-abdominal DT’s are rarer still and are often associated with FAP (of which our patient had no family history or endoscopic evidence of). They pose diagnostic difficulties as radiologically they closely resemble GISTs, leiomyosarcoms and other soft tissue neoplasms. Transabdominal biopsies are often not considered due to the risk of seeding a potential GIST [7]. Even with a tissue sample there remains difficulties in histologically diagnosing DTs.
Intra-abdominal DT’s also pose major therapeutic difficulties. As the incidence of DTs is so low there currently exists no evidence based or widely accepted guidelines on how to manage unresectable DT’s.
Because of the infiltrative pattern of DTs and their often encasement of the mesenteric vessels, complete R0 resection is often not possible.
The arsenal of systemic agents deployed against DT’s is broad owing to the scarcity of trials in the area. Broadly speaking options can be classified into cytotoxic and non-cytotoxic targeted agents. The choice of initial therapy takes into consideration the patients clinical condition, the rate of growth of the DT and the involvement of major vessels [8].
Whilst DT are classified as benign lesions we hope this case report highlights the diagnostic and therapeutic hurdles they pose. These lesions can be incredibly infiltrative rendering them inoperable. Should they continue to grow they will threaten vital organs and ultimately the patient’s life. More research is needed to identify the underlying pathophysiology of these lesions with the aim of providing more targeted therapies for their treatment.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the valuable assistance of Dr Jane Thorne, Consultant histopathologist Our Lady Of Lourdes Hospital Drogheda, and the department of pathology Our Lady of Lourdes Hospital Drogheda in completing this article.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- abdominal pain
- colonoscopy
- upper gastrointestinal endoscopy
- china
- dyspepsia
- fibromatosis, aggressive
- intestine, large
- intestine, small
- laparotomy
- mesentery
- surgical procedures, operative
- abdomen
- diagnosis
- histology
- small-intestine resection
- cytokinesis
- colectomy, right
- abdominal ct
- transverse colon
- dishonesty
- left upper quadrant of abdomen
- early satiety
- soft tissue mass



