-
PDF
- Split View
-
Views
-
Cite
Cite
V Vishnoi, P Liebenberg, F Reid, A Ward, B Draganic, A primary germ cell tumour in the gastrointestinal tract: a caecal lesion of yolk-sac morphology in a young patient, Journal of Surgical Case Reports, Volume 2018, Issue 11, November 2018, rjy291, https://doi.org/10.1093/jscr/rjy291
Close - Share Icon Share
Abstract
A 24-year-old man with a history of Crohns disease, whilst undergoing surveillance colonoscopy was found to have an ulcerated caecal lesion. The histopathology from the mucosal biopsy was suggestive of a yolk sac tumour. After thorough re-examination, the patient had no radiological evidence of malignancy in his testes or retroperitoneum. His alpha-fetoprotein levels returned as 2145, whilst his carcinoembryonic antigen was negligible. The patient was therefore consented for and underwent a laparoscopic right hemi-colectomy with an ileocolic anastomosis, without any complications. The formal histopathology confirmed the results from the biopsy, of a yolk sac non seminous germ cell tumour with positive lymph nodes and lymphovascular invasion. The patient was referred on to medical oncology for neoadjuvant chemotherapy. As the literature in his instance is scarce, the patient’s overall prognosis remains unclear. To the best of our knowledge this is the first reported primary germ cell tumour of the gastrointestinal tract.
CASE REPORT
We present a 24-year-old male with a background of Crohn’s disease. He was diagnosed with Crohn’s disease following a colonoscopy demonstrating macroscopic evidence of active inflammation in the colon, which was confirmed histologically. This was in the context of a prolonged history of periodic abdominal pain, weight loss and chronic diarrhoea. He was subsequently commenced on appropriate disease modifying anti-rheumatic drugs, resulting in complete remission. He otherwise has no other significant medical, surgical or family history. He is not a smoker and does not consume alcohol to excess. He is regularly followed by his gastroenterologist with screening endoscopies.
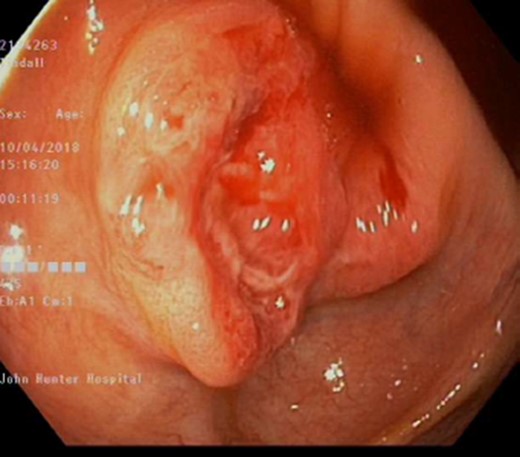
Apart from an occasional flare of his inflammatory bowel disease, requiring corticosteroids, he has been well. During his most recent screening colonoscopy he was found to have a 25 mm polypoid, sessile, friable, ulcerated lesion in his caecum (Fig. 1). The patient underwent a mucosal biopsy for further histological analysis. There was no other abnormality detected. This is in the context of no cardinal symptoms, and otherwise being completely asymptomatic.
The histopathology from the mucosal biopsy showed fragments of an ulcerating tumour, comprising nests, small dyshesive clusters and poorly formed tubules amongst desmoplastic stroma. Intravascular tumour emboli were present within small vessels. Also, there was fragments of large bowel mucosa showing villous accentuation, hypermucinosis and patchy infiltrates of neutrophils. These features were initially attributed to a likely invasive adenocarcinoma. Subsequent staining however was diffusely positive for glypican 3 and alpha feta protein (AFP). Thus, the final report concluded the polyp as a malignant lesion with differentials including invasive adenocarcinoma or metastatic germ cell tumour (GCT)/yolk sac tumour.
The patient underwent further work up with tumour markers, of which his AFP was positive at 2145 IU/ml. His other tumour markers including, carcinoembryonic antigen (CEA) and human chorionic gonadotropin (HCG) were negative. Given the unusual histopathology the patient was thoroughly reexamined looking for evidence of other lesions. Clinically the patient had no evidence of testicular masses, which was confirmed with a testicular ultra sound demonstrating no evidence of masses or lesions. A brain magnetic resonance image (MRI) was performed showing no evidence of pineal lesions. A positron emission tomography (PET) scan was performed showing increased fluorodeoxyglucose (FDG) uptake in the caecum, and nearby in the mesentery (medially) and possibly two small lymph nodes anterior to the caecum (Fig. 2). After discussion at a multi-disciplinary meeting with medical/radiation oncologists and surgeons, it was concluded that the primary was likely in the caecum. He was subsequently consented for a laparoscopic right hemi-colectomy.
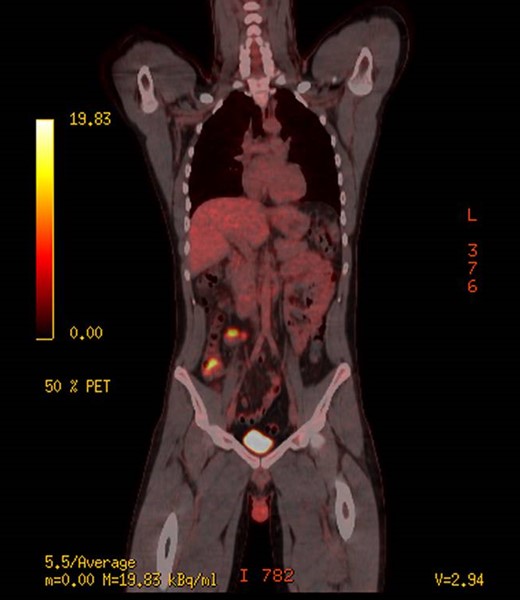
PET scan, demonstrating increased uptake in the caecum and likely mesentery lymph nodes.
The operation was performed with a primary ileocolic anastomosis. The histopathology was reported as a 28 mm tumour involving the mucosa, submucosa and the inner layer of the muscularis propria of the caecum. The tumour displayed a predominately solid architecture with minor components of glandular microcystic and hepatoid patterns. The tumour cells were high grade and large with atypical hyperchromatic nuclei and clear to eosinophilic cytoplasm. Immunostaining again positive for Glypican 3 and AFP (Fig. 3), thus confirming a GCT.
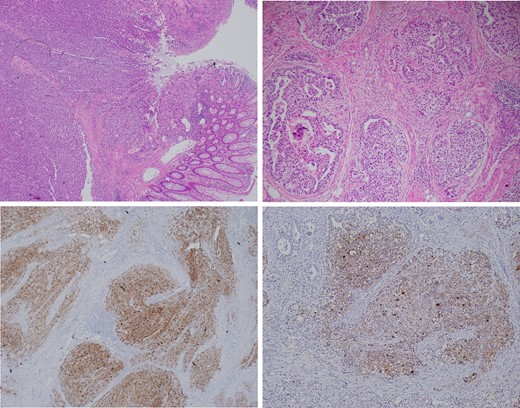
Top left: A low power view of colonic mucosa giving way to the solid tumour mass which involves the mucosa, submucosa and muscularis propria. Top right: A high-power view of the primitive appearance of a YST showing glomeruloid, microcystic and solid architecture and high-grade cytology. Focal necrosis is also observed. Bottom left: The tumour shows diffuse immunohistochemical staining for Glypican 3. Bottom right: The tumour shows diffuse immunohistochemical staining for AFP.
The patient recovered well from surgery and was discharged Day 3 post operatively. He was referred on to the local medical oncology team for commencement of neoadjuvant chemotherapy.
DISCUSSION
GCTs classically occur in either the testes or ovaries (90–95%) [1]. The pathogenesis involves neoplastic transformation in either mature cell lines or embryonal lines. The former gives rise to ovarian cystic teratomas as one example. Embryonal origin GCTs occur in a younger demographic and are more aggressive, often associated with metastatic disease at time of diagnosis [2]. Examples include choriocarcinoma and dysgerminoma.
Extragonadal disease is defined by the presence of a GCT in the absence of a primary mass in the testes or ovary (1–5%) [2]. Extragonadal GCTs are rare and typically arise in midline locations, specific sites vary with age. In adults, the most common sites, in order of frequency include the anterior mediastinum, the retroperitoneum, pineal and suprasellar regions [3]. In infants and young children, sacrococcygeal and intracranial GCTs are more common [4]. Extragonadal GCTs are more frequently seen in young patients with different histological subtypes and locations. Generally, germ cell tumours have a good prognosis, whereas extragonadal GCTs have a poor prognosis. Stage, site of origin, histologic type and high tumour burden are the most important prognostic factors. The treatment requires a multimodality approach. The true pathogenesis of extragonadal GCTs is as yet undetermined. One theory suggests that primordial germ cells that may have been arrested along the migratory route from the hindgut yolk sac region into the embryonic genital ridge; this would account for many extragonadal germ cell neoplasm’s arising in the midline [5].
An extragonadal GCT of gastrointestinal origin is rare, most commonly it is secondary to metastatic disease from a retroperitoneum primary or undetected testicular primary [6]. The majority of patients with gastrointestinal disease (either primary or metastatic), often have symptoms such as epigastric pain, nausea, vomiting, GI bleeding, iron deficiency anaemia which warrants further investigation. Our patient presented completely asymptomatic, in fact was undergoing a routine surveillance colonoscopy as part of his Crohn’s disease. Histologically and radiologically he was confirmed to have a primary caecal GCT, which to the best of our knowledge is the first reported in the literature. Following the patients right hemi-colectomy, the management will now be heavily dictated by the medical oncology team. As the literature in this instance, is scarce, the type and duration of chemotherapy will be difficult to determine.
Conflict of Interest statement
None declared.
References
Author notes
V. Vishnoi and P. Liebenberg The corresponding author is not a recipient of a research scholarship. This article is not based on a previous communication to a society or meeting.
- alpha-fetoproteins
- biopsy
- cancer
- colectomy
- colonoscopy
- crohn's disease
- ulcer
- germ cell tumor
- yolk sac tumors
- laparoscopy
- medical oncology
- retroperitoneal space
- yolk sac
- carcinoembryonic antigen
- cecum
- lymph nodes
- mucous membrane
- testis
- gastrointestinal tract
- surveillance, medical
- ileocolic anastomosis
- lymphatic invasion
- chemotherapy, neoadjuvant
- histopathology tests



