-
PDF
- Split View
-
Views
-
Cite
Cite
Peter Rogers, Corinne Jones, Andrew Dean, A rare skin condition masquerading as a serious wound infection, Journal of Surgical Case Reports, Volume 2018, Issue 11, November 2018, rjy299, https://doi.org/10.1093/jscr/rjy299
Close - Share Icon Share
Abstract
Pyoderma gangrenosum is a rare, serious and commonly missed condition that can effect post-operative surgical patients. The condition is commonly misdiagnosed as a wound infection, with subsequent inappropriate antibiotic therapy and wound debridement. We present the case of a 46-year-old patient who suffered this delayed diagnosis and multiple unnecessary interventions. We present this in an effort to raise awareness of the rare but serious condition, pyoderma gangrenosum.
INTRODUCTION
We present the case of a 46-year-old nulliparous lady, referred for management of wound infection post left breast lump excision. Past medical history was notable for prior chemotherapy for Histiocytosis X, pulmonary fibrosis, severe myelodysplasia, pleural cysts, severe pulmonary hypertension and a BMI of 38.
CASE REPORT
The patient presented with left nipple discharge in December of 2015. A 4 cm shadow in the left breast was identified on mammogram, with ultrasound commenting on a 9 mm left retro-areolar mass. FNA showed a papillary neoplasm. She underwent a left breast wire guided excision biopsy (5 May 2016). This was complicated by a wound haematoma that was drained 4 days later.
The excised tissue revealed a 6 mm papillary lesion and grade 1 multifocal ductal carcinoma within 32 mm of low to intermediate grade DCIS. This corresponded to the shadow on the mammogram. The cancer was strongly ER positive (>95%), PR positive (90%) and HER2 negative.
On the seventh post-operative day, the breast was found to be grossly oedematous, hard, with surface ulceration, pupura and extensive cellulitis. The patient was in severe pain, tachycardic, sweating and febrile despite broad spectrum antibiotics for 48 h and therefore taken to theatre. In theatre the breast and wound edges were rigid and peeling with extensive bruising, solid clot was drained from the wound which was then extended, debrided irrigated and packed. Marrow dysfunction secondary to myelodysplasia was considered a major complicating factor.
There was minimal clinical improvement in the post-operative period, significant pain and wound erythema continued. Two days later she returned to theatre for wound debridement and irrigation for presumed ongoing wound cellulitis (Fig. 1).
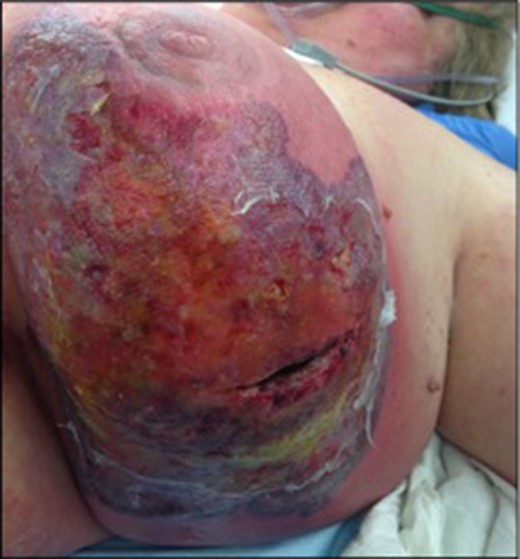
Extensive cellulitis with superficial ulceration post second drainage and debridement.
Due to the poor state of the wound, the extensive breast cancer, the underlying immune suppression and multi-disciplinary team discussion, completion left mastectomy with axillary node sampling was performed on the 25 May 2016. The wound was closed under tension with a 5 cm skin defect at the medial end of the scar. Pathology revealed no further invasive malignancy.
Repeated wound swabs continued to culture Staph Epidermidis and Staph Aureus with CRP fluctuating from 100 to 250.
Over the next 48 h, due to wound deterioration, the patient was taken to theatre a further two times. The mastectomy stitches were divided, the wound opened and once again debrided (Fig. 2).
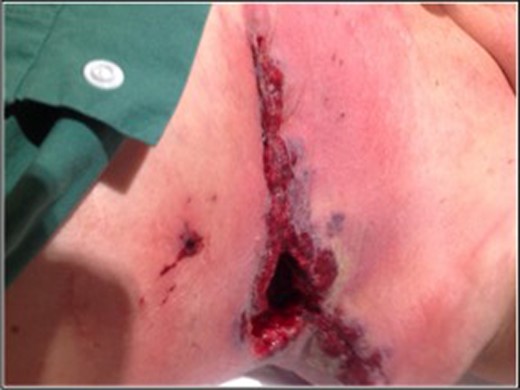
Image taken prior to mastectomy stitches being cut, wound shows granulation tissue and classic violaceous border of pyoderma gangrenosum.
A plastic surgeon and a dermatologist attended a return to theatre (1 June 2016) and suggested pyoderma gangrenosum being the cause for wound deterioration. Prior wound histology reported as inconclusive was reviewed by the dermatopathologist confirming pyoderma gangrenosum.
The next day, IV antibiotics were ceased with high dose steroids and cyclophosphamide commenced. Within 48 h of immunotherapy, the wound had significantly improved in terms of pain, erythema and swelling. The CRP reduced and the patients overall health dramatically improved. After significant resolution of wound inflammation, the patient underwent a full thickness skin graft of the left breast (Fig. 3).
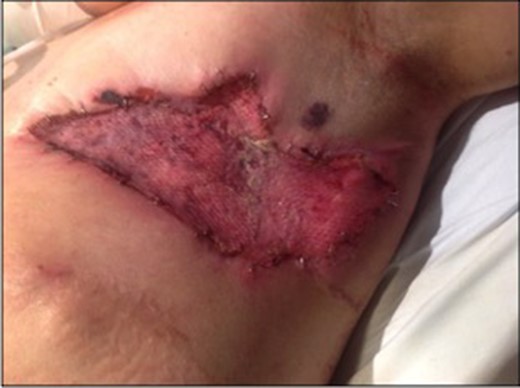
Post skin graft recovery was unremarkable and the patient was discharged home after more than 100 days of admission. Immunotherapy was continued on discharge.
DISCUSSION
Pyoderma Gangrenosum can be characterized as a chronic inflammatory dermatosis. The pathophysiology of the condition is not entirely understood, but it is thought to occur as a result of abhorrent trafficking of neutrophils and interleukins with suggested auto-immune dysfunction [1]. Much more commonly, it is seen in conjunction with other conditions such as inflammatory bowel disease, haematological dysfunction and rheumatic disease [2]. Classically the condition presents 7 days post procedure with erythema at the surgical site and pain out of proportion to clinical signs. Frequently the wound edges are described as dusky red, purplish [3], or with a violaceous border. Eventually the erythema may progress to wound dehiscence, at which point wound infection is generally diagnosed [4].
In a study by the Mayo Clinic, 122 patients with post-operative PG were reviewed [5]. Of those cases, the most common surgical sites for disease were breast and abdomen, with the most frequent comorbidity haematological dysfunction followed by inflammatory conditions and IBD. In 122 cases prior to PG diagnosis, 90% received empirical antibiotics and surgical debridement was performed in 73%, an alarmingly high percentage of inappropriate medication and procedures.
In conclusion, post-operative pyoderma gangrenosum is a rare, but serious wound complication. We present this case in an attempt to raise awareness of the condition, as failure of early recognition can lead to multiple unnecessary surgeries that can significantly increase patient morbidity.
ACKNOWLEDGEMENTS
Mr and Mrs Kennedy.
CONFLICT OF INTEREST STATEMENT
None declared.
DECLARATION
The corresponding author has received no research scholarship. This submission has not been concurrently communicated with any other journal or association.
REFERENCES



