-
PDF
- Split View
-
Views
-
Cite
Cite
James Jie Min Lam, Francesco Di Maggio, William Lynn, David Khoo, Oesophageal adenocarcinoma following gastric band surgery in two patients, Journal of Surgical Case Reports, Volume 2018, Issue 10, October 2018, rjy293, https://doi.org/10.1093/jscr/rjy293
Close - Share Icon Share
Abstract
Oesophageal adenocarcinoma following gastric band surgery has only been reported three times previously. The incidence is higher in morbidly obese patients, and its pathogenesis is correlated to reflux-induced microenvironmental changes. Bariatric surgery is transformative and its potential benefit for a substantial population is huge. Although no causal relationship with bariatric procedures has been evidenced to date, symptoms of adenocarcinoma—particularly anorexia, weight loss and dysphagia—can easily be overshadowed by alterations in eating patterns associated with weight-loss procedures. We report two cases of oesophageal adenocarcinoma in patients who had undergone a gastric banding procedure, and invite readers to consider the role that pre- and post-operative acid reflux dynamics may have precipitating neoplastic disease, and how endoscopic surveillance may play a role in prevention.
INTRODUCTION
Oesophageal cancer is one of the most poorly understood, yet deadliest cancers recorded worldwide, owing to its aggressive nature and preponderance for advanced stage presentation [1]. UK mortality rate for oesophageal cancer is the highest in all of Europe. Adenocarcinoma subtype has significantly increased as a proportion of overall diagnoses in Western countries, rising from 10 to 40% [1].
The United Kingdom and much of the Western world continue to be embroiled in a worsening epidemic of obesity, a state of ill health with recognised genetic and environmental components [2], often complicated by associated conditions.
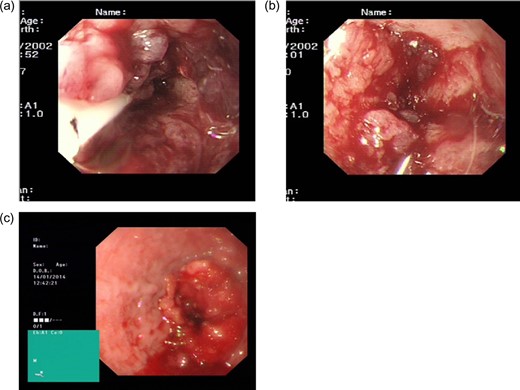
Gastroscopy findings. BH: (a) an area of thickening proximal to the gastro-oesophageal junction can be seen, BH: (b) with considerable oedema and dilatation of the oesophagus. PM: (c) a malignant stricture identified at 35 cm, with overlying friable mucosa.
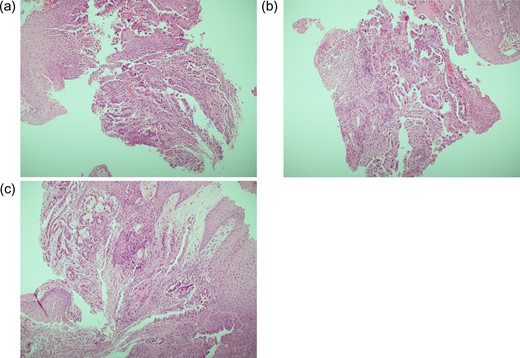
Histology findings. PM: (a–c) distal oesophagus biopsies, including at the level of the oesophageal stricture described in Fig. 1. Diffuse infiltration of stromal tissue by poorly differentiated adenocarcinoma is seen; parts of tumour exhibit tubule formation, and are lined by columnar cells which themselves demonstrate pleomorphic nuclei and epithelial tufting.
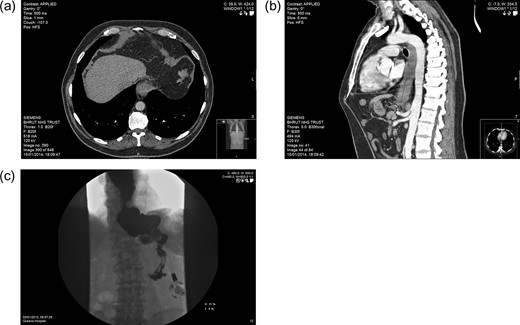
Imaging. PM: (a) axial CT section showing hypodensity in segment VIII of the liver, likely representing metastasis. BH: (b) Sagittal CT section demonstrating features of malginancy in the distal third of the oesophagus; dilatation of the middle and upper parts is notable owing to distal stenosis, and a soft tissue-density lesion is apparent in the final 5 cm. BH: (c) barium swallow study showing stasis in the distended lower oesophagus, with almost no contrast observed entering the stomach.
Bariatric surgery is the most cost-effective approach to treating severe obesity and its correlates, particularly in terms of long-term sustainability, with meta-analysis indicating improvements in mortality, weight reduction and glycaemic control far in advance of what remains achievable through non-surgical strategies [3, 4].
It has been recommended that the NHS re-evaluates its position regarding the use of bariatric surgery, with some reports advising 50 000 additional procedures a year to meet the health needs of the UK population [5]. It therefore becomes increasingly prudent to constantly reassess the long-term safety of these interventions.
The occurrence of oesophageal adenocarcinoma on post bariatric procedures patients has been reported in other instances [9, 10], with endoscopic and histological evidence of Barrett’s metaplasia being present as a precursor to malignant change.
The question of whether this patients should be followed up with long-term surveillance remains topical.
CASE 1
BH was a 66-year-old male admitted with worsening dysphagia. His past medical history included a gastric band insertion 10 years previously and longstanding diabetes with peripheral neuropathy dating back to before the banding procedure.
He had been an otherwise fit gentleman with a WHO performance status of zero. He had presented on several occasions with band adjustment issues, alternating between complete lack of restriction and near-complete obstruction, even with relatively small variations in band fluid volume.
His dysphagia was indeed initially thought to be band volume-related; however, when the band was deflated the swallowing problem persisted.
A subsequent water-soluble swallow test indicated satisfactory band position, but a significant irregularity of the distal oesophagus, just proximal to the band, with a substantial hold up of contrast at this level (Fig. 3c). Further investigations were duly arranged.
Gastroscopy revealed a dilated fluid filled oesophagus and considerable thickening at the gastro-oesophageal junction, with possible invasion of the diaphragmatic crux (Fig. 1a,b).
The following CT scan showed a 5 cm long thickening of the lower oesophagus extending to the gastro-oesophageal junction, with nodal involvement in segments 2 and 5 (Fig. 3b). Further hypodense metastatic lesions were identifiable in the liver. There was also evidence of peritoneal disease, in the left para-colic gutter and right supra-hepatic space. Tissue biopsies confirmed a poorly differentiated intestinal-type adenocarcinoma. It was subsequently arranged for BH to have a stenting procedure to relieve his dysphagia, and palliative chemotherapy.
CASE 2
PM, a 58-year-old male, had a gastric band inserted in 2011, after conservative efforts to resolve his morbid obesity failed. He was followed up on two separate occasions in 2012, and by August had lost 30 kg, with an oral intake of three regular meals. The surgical procedure had been successful, with band adjustment deemed unnecessary at either clinical encounter.
In December 2013 PM presented with a short history of progressive dysphagia, initially thought to be related to the gastric band. The symptoms persisted following removal of the band, and the patient proceeded to have a gastroscopy, which revealed a malignant stricture of the distal oesophagus (Fig. 1c).
Biopsies indicated a poorly differentiated adenocarcinoma (Fig. 2). CT scan suggested metastatic deposits in the liver and right kidney, alongside lymph node irregularities in coeliac axis and gastric lesser curve (Fig. 3a). A PET scan demonstrated localised regional uptake. The patient was commenced on palliative treatment, which included an endoscopic stent to the lower oesophagus and EOX chemotherapy. Prior to his diagnosis of cancer, PM only suffered from hypertension. His performance status at the time of commencing palliative chemotherapy was 1. Sadly he passed away shortly before he was due to commence cycle 2 of chemotherapy.
DISCUSSION
Fewer than 30 cases of oesophageal or gastric adenocarcinoma following bariatric surgery have been reported [6–8]. Of these, only five described adenocarcinoma of the oesophagus or gastro-oeseophageal junction, with this manuscript constituting the fourth and fifth description of adenocarcinoma of the oesophagus following gastric banding. Worldwide, over 400 000 gastric bands have been inserted in the past two decades: numbers indicate a direct causal relationship to be unlikely.
What is perhaps more notable is the higher rates of gastro-oesophageal reflux disease among morbidly obese patients, and of acid-induced premalignant condition recognised as Barrett’s oesophagus [1, 9].
A BMI >25 has been associated with significantly increased risk for oesophageal or cardial carcinoma [10]. Bariatric population is therefore presumably predisposed to an increased risk, although there is also potential for lead time bias, as premalignant or malignant changes may be noticed earlier with pre-operative assessment.
Dysplastic changes remain the only reliable factor for identifying patients at increased risk for oesophageal adenocarcinoma [1].
Notably, a significant factor potentially underlying the advanced presentation in both our cases was a degree of diagnostic overshadowing by band-related symptomatology. These cases also highlight the substantial variations in time from the procedure to the presentation of malignant disease, as reflected in other studies (Table 1).
Reports of patients with oesophageal or gastro-oesophageal junction adenocarcinoma following bariatric surgery, with time from operation to diagnosis of de novo cancer.
| Case . | Sex . | Age . | Procedure . | Years after procedure . |
|---|---|---|---|---|
| 1 | Female | 50 | Gastric band | 8 |
| 2 | Male | 43 | Gastric band | 2 |
| 3 | Male | 66 | Gastric band | 10 |
| 4 | Male | 58 | Gastric band | 3 |
| 5 | Female | 57 | Jejunoileal bypass | 26 |
| 6 | Vertical banded gastroplasty | 16 | ||
| 7 | Male | 54 | Roux-en-Y | 21 |
| 8 | Male | 50 | Roux-en-Y | 14 |
| Case . | Sex . | Age . | Procedure . | Years after procedure . |
|---|---|---|---|---|
| 1 | Female | 50 | Gastric band | 8 |
| 2 | Male | 43 | Gastric band | 2 |
| 3 | Male | 66 | Gastric band | 10 |
| 4 | Male | 58 | Gastric band | 3 |
| 5 | Female | 57 | Jejunoileal bypass | 26 |
| 6 | Vertical banded gastroplasty | 16 | ||
| 7 | Male | 54 | Roux-en-Y | 21 |
| 8 | Male | 50 | Roux-en-Y | 14 |
Reports of patients with oesophageal or gastro-oesophageal junction adenocarcinoma following bariatric surgery, with time from operation to diagnosis of de novo cancer.
| Case . | Sex . | Age . | Procedure . | Years after procedure . |
|---|---|---|---|---|
| 1 | Female | 50 | Gastric band | 8 |
| 2 | Male | 43 | Gastric band | 2 |
| 3 | Male | 66 | Gastric band | 10 |
| 4 | Male | 58 | Gastric band | 3 |
| 5 | Female | 57 | Jejunoileal bypass | 26 |
| 6 | Vertical banded gastroplasty | 16 | ||
| 7 | Male | 54 | Roux-en-Y | 21 |
| 8 | Male | 50 | Roux-en-Y | 14 |
| Case . | Sex . | Age . | Procedure . | Years after procedure . |
|---|---|---|---|---|
| 1 | Female | 50 | Gastric band | 8 |
| 2 | Male | 43 | Gastric band | 2 |
| 3 | Male | 66 | Gastric band | 10 |
| 4 | Male | 58 | Gastric band | 3 |
| 5 | Female | 57 | Jejunoileal bypass | 26 |
| 6 | Vertical banded gastroplasty | 16 | ||
| 7 | Male | 54 | Roux-en-Y | 21 |
| 8 | Male | 50 | Roux-en-Y | 14 |
It is therefore worth considering whether post bariatric procedures patients should have a lower threshold for endoscopic surveillance.
CONCLUSIONS
Bariatric surgery has the potential to transform lives, with implications for the health and well being of individuals, as well as of society as a whole.
Numbers are set to increase, and it is worth remembering that patients in whom surgery is successful are not without long-term risks.
Malignancy can present insidiously, as common presentation patterns such as weight loss, anorexia and dysphagia can easily be masked by alterations in eating habits following the procedure.
A degree of vigilance, a low threshold for endoscopy, and a recognition of the increased risk of oesophageal malignancy are required if we are to ensure that the health of these patients remains enhanced over the long term.
CONFLICT OF INTEREST STATEMENT
None declared.



