-
PDF
- Split View
-
Views
-
Cite
Cite
Seima Ohira, Hayato Ise, Sentaro Nakanishi, Daita Kobayashi, Ayumi Date, Shinsuke Kikuchi, Natsuya Ishikawa, Naoyuki Hasebe, Hiroyuki Kamiya, A left ventricular assist device for a patient with peripartum cardiomyopathy, Journal of Surgical Case Reports, Volume 2018, Issue 10, October 2018, rjy285, https://doi.org/10.1093/jscr/rjy285
Close - Share Icon Share
Abstract
Since its introduction in Japan in 1980, the extracorporeal left ventricular assist device has been used as a bridge to the recovery of cardiac function or to heart transplantation by many institutions. In this case report, we describe a 23-year-old female with peripartum cardiomyopathy. She had a persistently low cardiac index despite intensive care with intravenous inotropes, intra-aortic balloon pumping and extracorporeal membrane oxygenation; thus, we implanted an extracorporeal left ventricular assist device. Thereafter, her cardiac function gradually improved; the device was removed 2 months after the implantation. She currently has good heart function.
INTRODUCTION
Peripartum cardiomyopathy (PPCM), which shows a pathology similar to that of dilated cardiomyopathy, is a disease in which the development of heart failure is triggered by pregnancy in a woman without a history of heart disease. Here, we present a case of severe PPCM successfully treated with a left ventricular assist device (LVAD), which was used as a bridge to recovery.
CASE DESCRIPTION
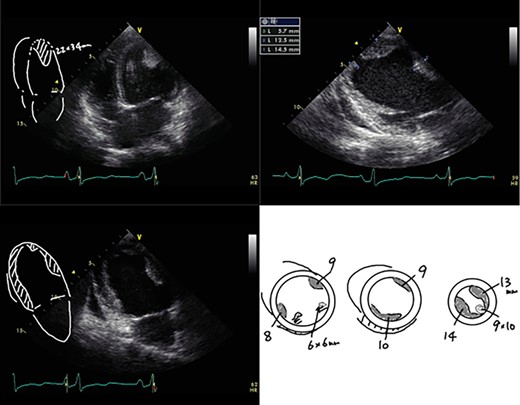
A 23-year-old woman presented with dyspnea after giving birth to her second child at a local hospital. Edema of both lower limbs gradually appeared, her labored breathing worsened, and the left ventricular ejection fraction (LVEF) was <30% by echocardiography. She was diagnosed with PPCM 5 months after childbirth. The LVEF had decreased to 10% and a left ventricular apical thrombus was also observed by echocardiogram (Fig. 1). She was transported to our institution due to progressive severe heart failure, and treated with intravenous inotropes, extracorporeal membrane oxygenation (ECMO) and intra-aortic balloon pumping (IABP). The LVEF subsequently decreased to 7% and left ventricular enlargement with multiple mural thrombi was observed. The condition was INTERMACS profile 1, and a LVAD was indicated. In Japan, an implantable LVAD can be applied only in patients in whom registration for heart transplantation has been already approved and the patient had no chance for an implantable LVAD. Thus, we implanted an external LVAD (AB 5000, ABIOMED, Danvers, MA, USA).
After the surgery, she received medical therapy consisting of carvedilol, 25 mg daily; enalapril, 5 mg daily; and bromocriptine, 5 mg daily for 2 weeks and 2.5 mg daily for 6 weeks, and her heart failure was controlled using furosemide, spironolactone and tolvaptan in combination. Aspirin (100 mg/day) and Warfarin (PT-INR 2.5-3.0) was also administered. Although her PT-INR had been strictly controlled within the therapeutic range, she had suffered from minor strokes several times. The N-terminal pro-brain natriuretic peptide level (NT-proBNP) improved from >5000 to <200 pg/ml in 2 months, and LVEF accordingly improved to 30%. She was smoothly recovering from wheel chair sitting position to walking through daily rehabilitation trainings. Based on her cardiac and general improvement and refractory minor strokes, the heart team decided to explant the LVAD. A LVAD withdrawal test was performed 2 months after the surgery, indicating that turning off the LVAD did not significantly affect cardiac function as well as water loading. NT-proBNP level temporarily increased but then gradually improved, and cardiac function also recovered to LVEF 42% after the LVAD removal (Fig. 2). One month later, she was transferred to a local hospital to continue her rehabilitation and finally discharged home. At 1 year after the symptom onset, she is suffering from NYHA II heart failure, but she was able to do normal daily activities, including raising her children. She gave informed consent for the publication of this report.
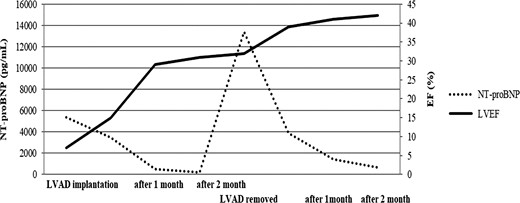
Serum NT-proBNP levels and the left ventricular ejection fraction (LVEF).
DISCUSSION
Here, we successfully treated a patient with severe heart failure due to PPCM using an extracorporeal LVAD. PPCM is a type of heart failure that develops upon pregnancy or childbirth; the estimated incidence of the disease is ~1 per 3000–4000 live births worldwide [1, 2]. In Japan, the disease occurs in ~1 in 15 000 pregnancies [3]. According to the report from USA, ~60% of patients with PPCM showed improvements in their cardiac function, and the remaining 40% experienced cardiac functional deterioration; 10% of the patients with the most severe disease experienced further worsening of their cardiac function and required heart transplantation [4]. Isogai investigated 283 PPCM patients hospitalized between 2007 and 2014; 4 patients (1.4%) died, 13 patients (4.6%) required mechanical circulatory support (12 IABPs and 5 ECMOs) and no patients received an LVAD [5]. Seguchi et al. [6] showed clinical results of 23 patients with severe heart failure with INTERMACS profile 1, indicating that only one patient with PPCM needed a BiVAD.
In the present case, we implanted only an LVAD, as our patient did not need a right ventricular assist device. Early LVAD implantation may be helpful in preventing further deterioration of the right ventricle. Although the external LVAD could be withdrawn successfully, she is suffering from chronic heart failure in NYHA II status. Careful follow-up is mandatory for her.
In conclusion, we experienced a case of severe PPCM treated with an LVAD as a bridge to recovery. PPCM is a rare disease, but severe cases can be fatal. Early LVAD implantation may be effective in patients with severe disease.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None declared.



