-
PDF
- Split View
-
Views
-
Cite
Cite
Henry To, Bee Shan Ong, Tom Dodd, Subhita Prasannan, Synchronous malignant phyllodes tumour and invasive lobular carcinoma—case report and review, Journal of Surgical Case Reports, Volume 2018, Issue 10, October 2018, rjy258, https://doi.org/10.1093/jscr/rjy258
Close - Share Icon Share
Abstract
Synchronous phyllodes tumour and invasive lobular carcinoma is an extremely rare event. We report these concurrent diagnoses in a patient observed in an ipsilateral breast, suspected due to breast risk factors (family history and lobular carcinoma in situ) and the presence of malignant phyllodes. Screening breast magnetic resonance imaging was able to identify the carcinoma which was occult in other imaging. An understanding of the possibility of dual diagnoses may lead to additional investigations for its identification. Treatment may then be tailored to the individual’s pathology.
INTRODUCTION
Phyllodes tumours (PT) are rare fibroepithelial tumours of the breast with an epithelial and cellular stromal component, and account for 1% of all breast tumours [1]. It is believed to arise from the periductal stromal cells of the breast, different from the ductal or lobular tissue from where invasive breast carcinomas originate. They are classified into benign, borderline and malignant based on the histology features that include cellularity, cellular pleomorphism, stromal overgrowth, mitotic activity and infiltration at the tumour edge [2]. They have metastatic potential, often via the haematogenous route to lung, bone and abdominal viscera.
Breast carcinoma may be ductal or lobular in origin and have a separate pathological basis for development than PT. Synchronous carcinoma with (or within a) PT is rare. If observed, it can arise from either within the epithelial component of PT, or near the fibroepithelial tissue [3]. There are only three case reports of lobular carcinoma with PT, all of which were within the PT or at its margin [4–6]. If present, lobular or ductal carcinoma in situ is more often observed than invasive carcinoma. PT is not known to increase the risk of carcinoma in the surrounding breast tissue, although the malignant subtype of PT may have a higher co-existence of carcinoma in the ipsilateral [7] or contralateral breast [3].
We report an unusual case of a malignant PT in a 48-year-old woman, who also had synchronous peri-marginal LCIS and a separate focus of invasive lobular carcinoma with axillary metastases. We discuss issues around the decision-making process for synchronous PT and breast carcinoma, with review of published cases and treatment recommendations.
CASE REPORT
A 48-year-old asymptomatic woman presented to breast screen, where mammogram identified a 65 mm right breast lesion at 10 o'clock region. She was overweight and had sleep apnoea, diabetes mellitus, hypertension, dyslipidemia and subclinical hypothyroidism. She had a family history of breast cancer in two second degree relatives (paternal grandmother diagnosed at ~60 years and maternal aunt diagnosed 58 years). Mammogram reported a Tabar grade 3b relatively well circumscribed lesion in right breast measuring 65 mm located 10 mm from nipple at 10 o'clock (Fig. 1A and B) and no other lesions. There was no associated microcalcification but some non-specific irregularity laterally and around the mass. At ultrasound, the main lesion appeared avascular and no other breast lesions were identified in both breasts. Histopathology of the core biopsy showed an atypical fibroepithelial lesion with features suspicious of a PT.
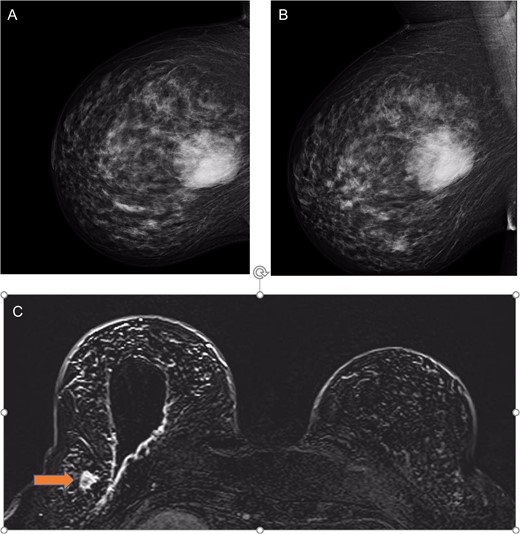
(a) Right mammogram cranial–caudal and (b) medial lateral oblique views showing the 65 mm phyllodes tumour; and (c) MRI T1 views with post-surgical wide local excision cavity and synchronous lobular carcinoma (labelled by orange arrow).
She underwent right wide local breast excision, where histopathological examination revealed spindle cell neoplasm favouring high grade (malignant) PT (Fig. 2A). Tumour is present at superficial and deep margins. Multi-disciplinary meeting discussion favoured mastectomy given the size of the lesion, but the patient opted for a re-excision. The decision was made for a sentinel node biopsy at the time of the re-excision, given the small but known lymph node metastasis rate in malignant PTs [8].
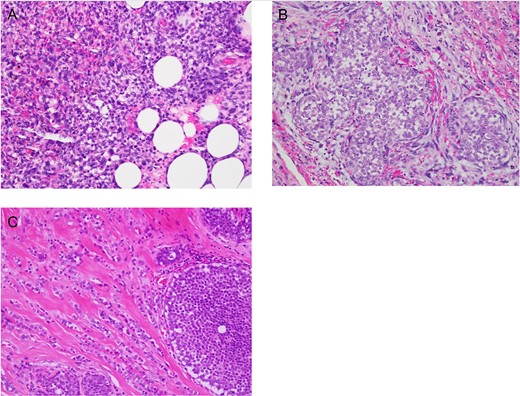
High power (×400) photomicrographs of Haematoxylin and Eosin stained slides showing (a) malignant phyllodes with infiltration beyond its margin to the breast fat, (b) lobular carcinoma in situ in margin next to malignant phyllodes and (c) lobular carcinoma with lobular carcinoma in situ.
Histological examination showed lobular carcinoma in situ within in the re-excision margin (Fig. 2B) with the right axilla sentinel lymph node identifying metastatic lobular carcinoma. On re-discussion at the multi-disciplinary team meeting, a mastectomy and axillary clearance was recommended. A screening breast MRI was performed to identify the mammographically occult lobular breast cancer and exclude a contralateral carcinoma. It showed post-operative changes and a 20 mm focal enhancement in the lateral right breast, 2 cm lateral to the surgical cavity, which was suspicious for a breast carcinoma (Fig. 1C).
Histopathology after the right total mastectomy and axillary clearance demonstrated a completely excised 15 mm grade II lobular carcinoma which was ER positive and Her-2 negative (Fig 2C). No residual PT was identified. In total, axillary lymph node assessment showed a macrometastasis in one of 34 lymph nodes. Chest wall and targeted nodal radiotherapy was recommended without the need for chemotherapy. Patient recovered well and surveillance would be continued in the outpatient breast specialist clinic with endocrine blockade therapy.
DISCUSSION
The case presented is the first reported of an exceedingly rare synchronous diagnosis of PT with associated peri-marginal lobular carcinoma in situ and a separate radiologically occult lobular invasive carcinoma with metastases. The synchronous diagnosis was identified after sentinel node biopsy, and was suspected based on breast risk factors, the presence of lobular carcinoma in situ and the reported association with malignant PT.
PT are classified into benign, borderline and malignant based on the histology features that include cellularity, cellular pleomorphism, stromal overgrowth, mitotic activity and infiltration at the tumour edge [2]. Aggressiveness may also be determined via their stroma profile, stromal call atypia, growth pattern (heterologous or homologous), lesion borders (well demarcated or infiltrative) and the presence/absence of necrosis [1, 9]. They have metastatic potential, often via the haematogenous (rather than lymphatic) route to travel direct to lung, bone and abdominal viscera. Staging via CT chest, abdomen and pelvis is recommended in borderline and malignant cases.
Surgical resection for PT (without axillary nodal assessment) is the mainstay of treatment, where a 10 mm margin is the recommended treatment [10]. Yearly clinical and radiological surveillance is then recommended, as local recurrence after treatment is common even in benign cases, observed at more than 8% of cases in 10 years [11]. Risk of recurrence is greater in borderline or malignant forms and is reported as high as 30% at 10 years [11].
On review of the literature to 2018, synchronous carcinomas are more often ductal and rarely lobular (Table 1). Reported ipsilateral carcinoma in situ or invasive carcinomas are almost all small and often found incidentally in the margins following resection of the dominant PT. The largest reported database of PT from China reported a 1.1% prevalence of carcinoma in situ or carcinoma with PT, stating a likely under-reporting in the general literature [7]. The same study noted that carcinoma was more often observed in malignant PT, although numbers were small. Of note, malignant PT was associated with poorer prognosis breast carcinomas (such as triple negative) and some patients had a past history of other cancers (without a known genetic syndrome) [4].
| Study . | Age . | Pathology . | Tumour location . | Surgical intervention . |
|---|---|---|---|---|
| Shirah et al. | 49 | Benign PT + ILC + LCIS | Within PT | Excisional biopsy |
| Kadoma et al. | 47 | Benign PT + ILC + LCIS | Within PT | Mastectomy |
| Potdiven et al. | 63 | Borderline PT + ILC and LCIS | Margin | Completion mastectomy and SLNB |
| Current case | 48 | Malignant PT + ILC + LCIS | Separate ipsilateral | Completion mastectomy and SLNB |
| Study . | Age . | Pathology . | Tumour location . | Surgical intervention . |
|---|---|---|---|---|
| Shirah et al. | 49 | Benign PT + ILC + LCIS | Within PT | Excisional biopsy |
| Kadoma et al. | 47 | Benign PT + ILC + LCIS | Within PT | Mastectomy |
| Potdiven et al. | 63 | Borderline PT + ILC and LCIS | Margin | Completion mastectomy and SLNB |
| Current case | 48 | Malignant PT + ILC + LCIS | Separate ipsilateral | Completion mastectomy and SLNB |
Abbreviations: PT = phyllodes tumour, ILC = invasive lobular carcinoma, LCIS = lobular carcinoma in situ; SLNB = sentinel lymph node biopsy
| Study . | Age . | Pathology . | Tumour location . | Surgical intervention . |
|---|---|---|---|---|
| Shirah et al. | 49 | Benign PT + ILC + LCIS | Within PT | Excisional biopsy |
| Kadoma et al. | 47 | Benign PT + ILC + LCIS | Within PT | Mastectomy |
| Potdiven et al. | 63 | Borderline PT + ILC and LCIS | Margin | Completion mastectomy and SLNB |
| Current case | 48 | Malignant PT + ILC + LCIS | Separate ipsilateral | Completion mastectomy and SLNB |
| Study . | Age . | Pathology . | Tumour location . | Surgical intervention . |
|---|---|---|---|---|
| Shirah et al. | 49 | Benign PT + ILC + LCIS | Within PT | Excisional biopsy |
| Kadoma et al. | 47 | Benign PT + ILC + LCIS | Within PT | Mastectomy |
| Potdiven et al. | 63 | Borderline PT + ILC and LCIS | Margin | Completion mastectomy and SLNB |
| Current case | 48 | Malignant PT + ILC + LCIS | Separate ipsilateral | Completion mastectomy and SLNB |
Abbreviations: PT = phyllodes tumour, ILC = invasive lobular carcinoma, LCIS = lobular carcinoma in situ; SLNB = sentinel lymph node biopsy
Summarizing the literature findings [4–6], there seem to be few pre-operative clinical indicators for this dual pathology. There does not appear to be any consistent risk factors or associations outside of breast cancer risks or past history of cancer. Review of radiology in multiple studies often have not reported significant breast stromal change that would suggest the co-existence of a carcinoma. Breast MRI was occasionally used to screen for other tumours. Overall, this is the first case reported where axillary metastasis was present with the synchronous lobular breast cancer.
In the reported case, the combination of a family history of breast cancer and mammographic changes in the breast permitted surgical axillary staging during re-excision. The positive sentinel node lead to the identification of a separate ipsilateral carcinoma focus. In addition, a breast MRI also allowed the screening of the contralateral breast for other breast masses and therefore can be used in such cases. Prognosis for synchronous patients are generally related to the carcinoma rather than the PT. As there if often early stage presentation of invasive carcinoma, surgery and adjuvant treatment provide a good prognosis.
There has not been any identified genetic relationship or common genetic pathways identified, although allelic loss a may be common mechanism for genetic alteration during tumorigenesis [12]. For local factors, the epithelial component of PT may have been stimulated by hormone and systemic growth factors, thus synchronously stimulating the growth of other surrounding atypical cells. Alternatively, the carcinoma may have had induction by the stromal component of the PT to affect the epithelium, the latter explaining why there may be an increase prevalence in malignant PT.
In conclusion, a rare ipsilateral synchronous diagnosis of lobular breast cancer may be considered in patients with breast risk factors in the presence of malignant PT. Imaging, such as breast MRI, may effectively identify the second primary cancer. Treatment via axillary staging and adjuvant therapy is required. Ongoing surveillance is the same for both diagnoses.
CONFLICT OF INTEREST STATEMENT
None declared.



