-
PDF
- Split View
-
Views
-
Cite
Cite
Mohamed S Kuziez, Daniel Picus, Luis A Sanchez, Mohamed A Zayed, Trans-luminal repair of a ruptured AAA with Type Ia and Type II endoleaks, Journal of Surgical Case Reports, Volume 2018, Issue 10, October 2018, rjy254, https://doi.org/10.1093/jscr/rjy254
Close - Share Icon Share
Abstract
Ruptured abdominal aortic aneurysm (rAAA) with an associated Type II endoleak is rare. Emergent surgical repair is often necessary and may be associated with high morbidity and mortality. We report an alternative unique trans-luminal repair strategy in an 84-year-old male who presented with a rAAA with prior EVAR, and Type Ia and Type II endoleaks. The operative strategy consisted of proximal endograft extension into the para-renal aorta, followed by staged sac embolization using glue. Postoperatively, the patient recovered well from the repair, and follow-up imaging demonstrated a stable repair.
INTRODUCTION
Endovascular aortic aneurysm repair (EVAR) is the most common method for repairing abdominal aortic aneurysms (AAA). Type II endoleaks following EVAR is common and can occur in up to 34% of repairs [1]. Although Type II endoleaks are commonly regarded as benign, in unique circumstances they can lead to continued aneurysm growth, and can rarely lead to sac rupture [2]. In this exceptional circumstance an emergent open aneurysmorrhaphy of the ruptured AAA (rAAA) is considered to be the most definitive treatment option. Here we report an alternative intra-luminal treatment strategy of a rAAA with a thrombus-seal Type Ia endoleak, and large Type II endoleak.
CASE REPORT
An 84-year-old male with a history of coronary artery disease, carotid artery stenosis, had EVAR 3 years prior for a 6.5 × 6.1 cm2 infrarenal AAA using a Excluder® bifurcated aortic endograft (W.L.Gore) at a different hospital. The patient presented to our hospital hemodynamically stable with 5 days of right flank/abdominal pains and mild anemia. A CTA of the abdomen revealed a large infrarenal aortic aneurysm sac measuring 8.9 × 9.7 cm2, with adjacent retroperitoneal hematoma and stranding (Fig. 1A). The juxta-renal aorta demonstrated mild aneurysmal degeneration with thrombus extending adjacent to the prior proximal aortic endograft fixation site, suggesting a proximal Type Ia thrombus-seal endoleak (Fig. 1B and C). Additionally, a large Type II endoleak was identified within the right lateral posterior aneurysm sac, adjacent to the aortic endograft, and originating from multiple lumbar arteries (Fig. 1D). The patient’s right kidney was small, atretic and non-perfused. The left renal artery was patent, and the left kidney was normal in size (Fig. 1B and E).
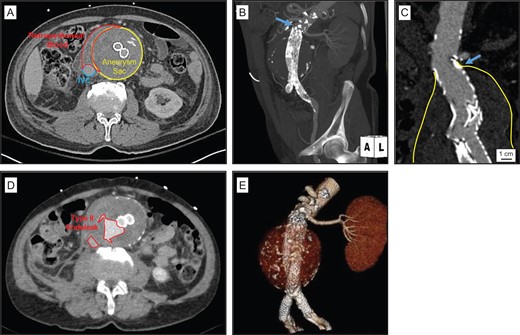
Ruptured AAA in the setting of large chornic Type II endoleak and inadequate proximal aortic endograft seal. (A) Emergency room CT demonstrated retroperitoneal stranding and blood consistent with aortic aneurysm rupture. (B) Left anterior oblique MIP reconstruction image demonstrates previously placed infrarenal modular bifurcated Gore® Excluder® aortic endograft, a single patent left renal artery, and mild aneurysmal degeneration in the juxta-renal aorta (blue arrow). (C) Coronal CTA demonstrates aneurysm sac extending to the level of the proximal Excluder® endograft directly below the level of the left renal artery (blue arrow). (D) CTA demonstrated large Type II endoleaks, adjacent to the level of the aortic aneurysm sac rupture site. (E) Left anterior oblique 3D reconstruction illustrates position of previously placed infrarenal aortic endograft and bilateral iliac limb extensions in the proximal common iliac arteries.
Given the patient’s advanced age and co-morbidities, an emergent open rAAA repair was considered too high risk. Alternatively we performed a staged trans-luminal repair of the thrombus-seal Type Ia endoleak, followed by a trans-lumbar aortic sac embolization of the Type II endoleak. First, a 10% oversized aortic endograft cuff (28 × 43 mm2 Zenith Renu®; Cook Medical) was placed below the inferior margin of the superior mesenteric artery and above the prior Excluder® endograft flow divider. A 6 × 38 cm2 iCASTTM stent (Atrium) was selected for snorkel stenting of the left renal artery (Fig. 2A). The Renu® aortic cuff and iCASTTM renal stent were deployed and simultaneously molded with balloon angioplasty to avoid a gutter leaks between the devices (Fig. 2B). Two AptusM EndoAnchorTM staples (Medtronic) were also deployed to fuse the distal Renu® cuff to the proximal neck of the Excluder® endograft, and provide additional active fixation to the para-renal aorta (Fig. 2C). Post-procedure the patient remained hemodynamically stable, and a CTA demonstrated a well-sealed para-renal aorta and patent snorkel left renal artery stent (Fig. 2E–G).
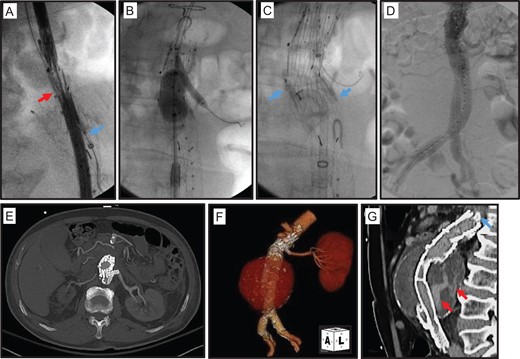
Extension of proximal aortic endograft seal to the para-renal aorta. (A) Placement of a Cook Zenith Renu® endograft cuff (red Arrow) from a right common femoral artery cannulation. A 7 Fr sheath (blue arrow) was also advanced into the proximal left renal artery from a left proximal brachial artery cannulation. (B) Molding of deployed Renu® cuff and a 6 × 22 mm2 AtriumTM iCASTTM stent in the proximal left renal artery. (C) Further fixation of deployed Renu® cuff with AptusTM EndoAnchorTM staples (blue arrow). (D) Completion aortogram demonstrates brisk flow in the aortic endograft and left renal artery. (E–G) Post-operative CTA demonstrates well positioned aortic endograft and adjacent snorkel left renal artery stent.
In a staged fashion the patient next underwent a trans-lumbar Type II endoleak embolization. The aortic sac was directly cannulated from a posterior left flank puncture. Aortic sac angiogram confirmed large Type II endoleaks from L3 and L4 lumbar arteries, and no opacification of the anterior spinal artery (Fig. 3A and B). The aneurysm sac was infused with a mixture of 1:2.5 N-butyl cyanoacrylate glue (NBCA) and Ethiodol. Repeat sac angiogram demonstrated complete stasis of flow within the aneurysm sac and feeding lumbar arteries (Fig. 3C).
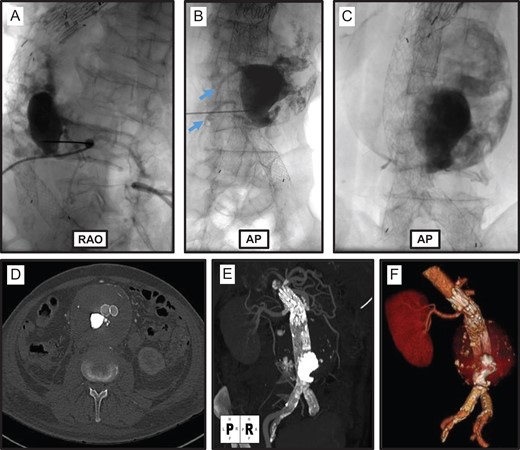
Trans-lumbar aortic sac embolization. Right anterior oblique (RAO; A) and anterior-posterior (AP; B) projections of trans-lumbar aortic puncture and angiogram demonstrating a large Type II endoleak originating from L3 and L4 lumbar arteries (blue arrows). (C) Completion fluorogram demonstrates NBCA/Ethiodol glue embolization of the Type II endoleak. (D–F) Post-procedure CTA demonstrates successful embolization of aortic sac Type II endoleak.
Within 24 h the patient’s pain resolved, and a CTA on post-procedure Day 3 demonstrated a stable proximal juxta-renal aortic repair, resolution of the Type II endoleak and thinning of the retroperitoneal hematoma (Fig. 3D–F). The patient was discharged home in a stable condition on Day 4. A CTA at 5 and 18 months post-procedure demonstrated continued stability of the juxta-renal snorkel repair, and gradual decrease in aortic aneurysm sac diameter to 8.4 × 9.5 cm2 (Fig. 4). At 1.5 years post-procedure the patient remains asymptomatic and physically active.
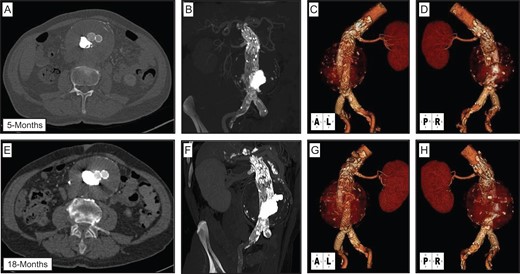
Post-operative follow-up CTA imaging. CTA performed at 5 months (A–D) and 18 months (E–H) demonstrates stable repair of aortic aneurysm, with continued aortic endograft and left renal artery stent patency, and no evidence of persistent endoleaks.
DISCUSSION
Here we report a unique presentation of a rAAA with a large Type II endoleak and thrombus-seal Type Ia endoleak. Although large chronic Type II endoleaks can lead to aneurysm sac expansion and rupture, this is rare and is estimated to occur in 4.5% of patients with endoleaks [1, 2]. Alternatively, progressive aneurysm degeneration of the juxta-renal aorta following EVAR can lead to inadequate proximal aortic neck Type Ia endoleak [3]. Either or both of these possibilities may have contributed to this patient’s gradual aneurysm sac expansion (>3 cm over 3 years), and ultimately rupture leading to retroperitoneal bleeding from lumbar arteries feeding the Type II endoleak.
There is only one prior report that demonstrates the feasibility of Type II endoleak embolization in the setting of a rAAA [4]. For this patient we decided that treatment of the Type Ia endoleak was first necessary since it would decrease the risk of glue dispersal into the aorta from the inadequate proximal seal. However, urgent endovascular repairs of the para-renal aorta pose unique logistical and technical challenges since there are currently no FDA-approved ‘off-the-shelf’ endografts for this segment of the aorta. Alternatively, a snorkel/chimney repair can be performed in the para-renal aorta with good results [5]. This patient’s right kidney was atretic and non-perfused, which allowed for a para-renal aortic repair with an aortic endograft cuff and a single snorkel renal artery stent. Follow-up evaluations demonstrated that the para-renal aortic repair was stable with no evidence of gutter endoleaks (Figs 3F, G and 4).
There are multiple treatment strategies for chronic Type II endoleaks [1, 6]. Patients who have an expanding AAA with a chronic Type II endoleak are acceptable candidates for attempted embolization [1, 7]. Access to the Type II endoleak can be achieved with a trans-lumbar or trans-caval aortic puncture, and aortic sac embolization can be achieved with the use of metallic coils or polymers [6]. Polymer-based embolization can be achieved with either Ethylene-Vinyl-Copolymer (OnyxTM) liquid or NBCA [8, 9]. In this patient, a standard trans-lumbar direct aortic sac puncture was performed, and glue was used to achieve sac embolization while reducing the risk of embolic reflux into the feeding lumbar arteries.
ACKNOWLEDGEMENTS
We thank Mrs Ronnie Eugea and Mrs Theresa Belgeri for assistance with this case report.
CONFLICT OF INTEREST STATEMENT
All authors of this article declare no conflicts in any regard. All authors of this article did not receive any financial support or award for this report.



