-
PDF
- Split View
-
Views
-
Cite
Cite
Takashi Miyata, Yusuke Yamamoto, Teiichi Sugiura, Yukiyasu Okamura, Takaaki Ito, Ryo Ashida, Sunao Uemura, Yoshiyasu Kato, Katsuhisa Ohgi, Atsushi Kohga, Tsuneyuki Uchida, Shusei Sano, Masahiro Nakagawa, Katsuhiko Uesaka, Pancreaticoduodenectomy with hepatic arterial revascularization for pancreatic head cancer with stenosis of the celiac axis due to compression by the median arcuate ligament: a case report, Journal of Surgical Case Reports, Volume 2018, Issue 1, January 2018, rjy002, https://doi.org/10.1093/jscr/rjy002
Close - Share Icon Share
Abstract
A 71-year-old woman presented to our hospital because pancreatic head cancer was suspected on a medical checkup. Computed tomography showed a 30 mm low-density lesion in the pancreatic head, and the stenosis of the celiac axis (CA) due to the median arcuate ligament (MAL) compression. We made a preoperative diagnosis of pancreatic head cancer and performed laparotomy. Transection of the MAL failed to restore adequate hepatic arterial flow, necessitating arterial revascularization, which was achieved by end-to-end anastomosis between the gastroduodenal artery and the middle colic artery. After reconstruction, Doppler ultrasonography showed improved hepatic arterial signal. The patient was discharged 16 days after surgery with no complications. When planning pancreaticoduodenectomy (PD) for such patients with CA stenosis due to MAL compression, surgeons should simulate a situation of insufficient hepatic arterial flow after division of the MAL, and prepare for reconstruction of the hepatic artery during PD.
INTRODUCTION
Celiac axis (CA) stenosis is not a rare condition [1], and compression by the median arcuate ligament (MAL) has been reported as its cause [2]. When performing pancreaticoduodenectomy (PD) in such patients, MAL division during surgery is recommended because CA stenosis influences the blood supply to the liver. However, in some cases, the hepatic arterial flow cannot be resolved by division of the MAL, and the diminished hepatic arterial flow induces hepatic ischemia [3]. To prevent hepatic ischemia, the surgical procedure, including the preservation of the pancreatic arcade [3] or revascularization of the hepatic artery [4], must be considered.
We herein report a case of pancreatic head cancer successfully treated by PD and hepatic arterial revascularization between of gastroduodenal artery (GDA) and middle colic artery (MCA) in a patient with CA stenosis due to MAL compression.
CASE REPORT
A 71-year-old woman was admitted because pancreatic head cancer was suspected on a medical checkup. Enhanced abdominal computed tomography (CT) revealed a low-density tumor, 30 mm in diameter, in the pancreatic head, and involvement of the superior mesenteric vein (SMV) with the tumor was noted (Fig. 1a). The distal side of the GDA was involved by this tumor, however, the proximal side of the GDA was not involved. CA stenosis was revealed (Fig. 1b). In addition, the developed arcade of the peri-pancreatic arteries and an aneurysm of the inferior pancreaticoduodenal artery (IPDA), 20 mm in diameter, were found (Fig. 1c). We preoperatively diagnosed the patient with pancreatic head cancer with CA stenosis due to MAL compression and aneurysm of IPDA, T3N0M0 Stage IIA (UICC seventh). We planned PD and SMV resection and reconstruction with opening of the MAL. If the hepatic arterial flow was found to be inadequate after division of the MAL, arterial reconstruction by end-to-end anastomosis of the GDA and MCA was planned.
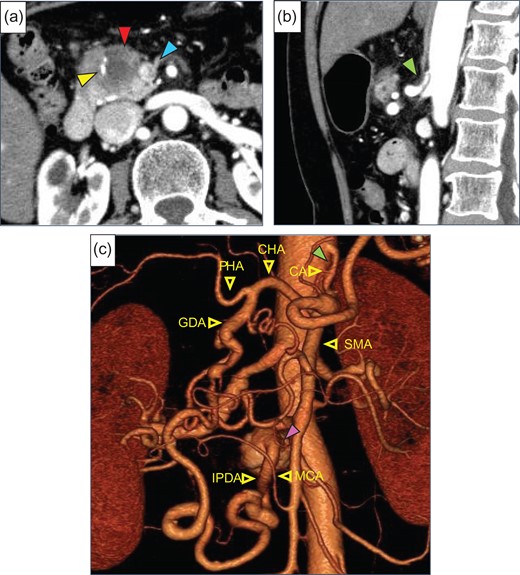
(a) Enhanced abdominal CT showed a low-density tumor (red arrow), 30 mm in diameter, in the pancreatic head. The superior mesenteric vein (SMV, blue arrow) and gastroduodenal artery (GDA, yellow arrow) were involved by this tumor. (b) Stenosis of the celiac artery (CA) was shown (green arrow). (c) Preoperative 3D CT angiogram showed the development of pancreatic artery arcade and a saccular aneurysm (purple arrow) of the inferior pancreaticoduodenal artery (IPDA), 20 mm in diameter. CHA, common hepatic artery; PHA, proper hepatic artery; SMA, superior mesenteric artery; MCA, middle colic artery.
After laparotomy we identified the MAL, which had compressed the origin of the CA (Fig. 2a), and Doppler ultrasonography showed that the hepatic arterial flow was hepatopetal, but the common hepatic arterial flow was hepatofugal (no record available). First, the MAL was divided (Fig. 2b). After clamping the GDA (Fig. 2c), the hepatic arterial flow was not satisfactory (Fig. 2d). We continued the operation under GDA clamping. Finally, the specimen was connected only by the IPDA, SMV and GDA. The IPDA and aneurysm were ligated and divided (Fig. 3a). At this time, 92 min after clamping the GDA, however, the hepatic arterial flow was not improved. We were able to preserve the GDA 10 mm from its root, we divided the GDA and SMV and extracted the specimen. Arterial reconstruction by end-to-end anastomosis of the GDA and MCA was performed by plastic surgeons (Fig. 3b). After reconstruction, the hepatic arterial signal was improved (Fig. 3c).
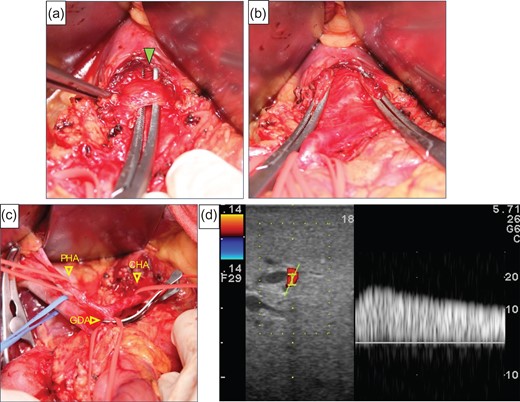
(a) The median arcuate ligament (green arrow) compressed the CA. (b) Compression of the origin of the CA by the ligament was released. (c) The image shows that the GDA was clamped. (d) We checked the hepatic artery flow under GDA clamping, but the flow had not improved satisfactory.
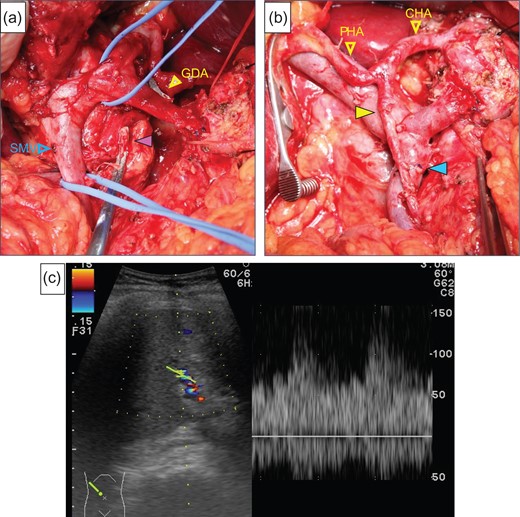
(a) The purple arrow indicates the stump of the aneurysm. (b) The image was after reconstruction by end-to-end anastomosis of the GDA and the MCA (yellow arrow). The blue arrow indicates the anastomotic part of the SMV. (c) We checked the hepatic arterial flow after reconstruction, the hepatic arterial signal had increased.
The patient was discharged 16 days after surgery with no complications. We made the final diagnosis of the pancreatic head invasive ductal carcinoma, T3N1M0 Stage IIB (UICC seventh). Adjuvant chemotherapy with S-1 was performed for 6 months. The patient did not develop recurrence in 14 months following the operation.
DISCUSSION
CA stenosis is not a particularly rare clinical entity, and a previous report in a large series described an incidence of 10.5% [1]. And the most common cause of CA stenosis in Eastern countries is the MAL [2]. CA stenosis is usually asymptomatic, due to the rich collateral mainly through the pancreaticoduodenal arcades. However, it is particularly critical to maintain the hepatic arterial flow when performing PD.
This method of preoperative stent insertion may be helpful for CA stenosis, and many reports of endovascular treatment have been published. This procedure can be fatal, however, if thrombosis or stent occlusion occurs [5]. MAL division may be able to resolve this issue and prevent problems, such as ischemic complications [5]. Therefore, in this case, preoperative transluminal angioplasty or stenting were not considered.
For CA stenosis due to MAL compression, MAL division is considered the primary procedure for surgical treatment. However, if the hepatic arterial flow cannot be resolved, surgeon should consider saving the pancreatic collateral arcade or revascularization of the hepatic artery. There have been several reports of simultaneous vascular reconstruction with PD for patients with CA stenosis due to MAL compression [1, 4, 6–8]. Simultaneous vascular reconstruction with PD carries an increased risk of thromboembolism and postoperative bleeding caused by pancreatic fistula [4]. While saving the pancreatic collateral arcade is one way of avoiding vascular reconstruction, preserving the collateral arcade carries a risk of losing positive surgical margins, particularly in cases of pancreatic head cancer. Furthermore, true aneurysms of the PDA are frequently coexistent with CA stenosis [9]. When PD is planned for patients with CA stenosis and aneurysms in the pancreatic arcade, surgeons often cannot preserve the pancreatic arterial arcade in radical surgery.
Several methods for surgical reconstruction of the hepatic artery have been reported. One is bypass grafting between such as aorta and CA, using a graft such as the saphenous vein [10]. And the other is end-to-end arterial anastomosis method such as our case. In bypass method, grafts extraction and two reconstructions are necessary. In contrast, end-to-end arterial anastomosis method could be achieved without the use of grafts. Previous reports have described anastomosis between GDA and MCA [4, 7], splenic artery and superior mesenteric artery [1], and MCA and right gastroepiploic artery [6]. Of these, end-to-end anastomosis between GDA and MCA, if both the MCA and the root of GDA were not involved by tumor, seems to be a viable and simple option in reconstruction during PD [4, 7].
When planning PD for the patients with CA stenosis, surgeons should simulate various situations for maintaining the hepatic arterial flow. Revascularization between the GDA and MCA is one way to maintain the hepatic arterial flow and allow for radical surgery. To our knowledge, although only two reports [4, 7] have applied this procedure with end-to-end anastomosis between GDA and MCA, the patient was able to be successfully treated in both cases.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING
No authors have direct or indirect commercial and financial incentives associated with publishing the article.
CONSENT
The patient discussed in this case report provided her informed consent for publishing the information in this report.
REFERENCES
- computed tomography
- acute abdominal pain
- doppler ultrasound
- health evaluation
- celiac artery
- constriction, pathologic
- hepatic artery
- laparotomy
- pancreaticoduodenectomy
- preoperative care
- reconstructive surgical procedures
- surgical procedures, operative
- diagnosis
- surgery specialty
- revascularization
- anastomosis, end to end
- malignant neoplasm of head of pancreas
- median arcuate ligament
- gastroduodenal artery
- pancreas head
- compression
- fluid flow



