-
PDF
- Split View
-
Views
-
Cite
Cite
Ikennah Browne, Pete Panayides, Azim Valji, Metastatic squamous cell cancer of the lung presenting as a perforated cecal cancer, Journal of Surgical Case Reports, Volume 2018, Issue 1, January 2018, rjx262, https://doi.org/10.1093/jscr/rjx262
Close - Share Icon Share
Abstract
Lung cancer is the leading cause of cancer deaths worldwide, with non-small cell lung cancer (NSCLC) accounting for 85% of diagnoses. Metastasis occurs in ~50% of cases but clinically evident isolated gastrointestinal (GI) metastasis is rare. We present a 78-year-old female who underwent an urgent right hemi-colectomy after cross-sectional imaging revealed a perforated cecal mass. Final pathology demonstrated squamous cell cancer of lung origin. We review the literature on NSCLC with clinically evident metastases to the GI tract, as well as important diagnostic considerations.
INTRODUCTION
Lung cancer is the leading cause of cancer deaths worldwide, with metastases detected in ~50% of cases [1–3]. Common sites include lymph nodes, adrenal glands, liver, bone, brain and contralateral lung [2, 3]. While symptomatic gastrointestinal (GI) metastasis of lung cancer is rare, occurring in 0.2–0.5% of cases, autopsy studies show metastatic disease to the GI tract to be far more common [3–5]. The incidence of isolated small bowel metastasis ranges from 7.5 to 19%, and that of isolated colonic metastasis from 2.5 to 5% [4, 5]. Authors theorize that the attribution of symptoms to chemotherapy may contribute to under-diagnosing GI metastases in lung cancer patients [3].
Nonetheless, symptomatic colonic metastasis of lung origin remains a rare entity. We report the case of a primary lung lesion with isolated colonic involvement presenting with perforation. In addition, we discuss the complexity of diagnostic considerations in this setting and the implications on management.
CASE REPORT
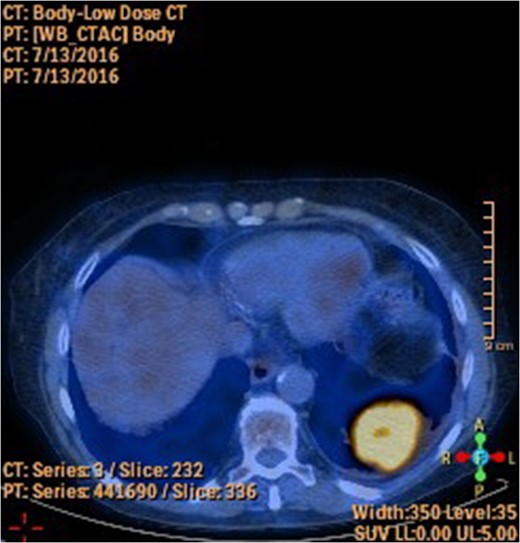
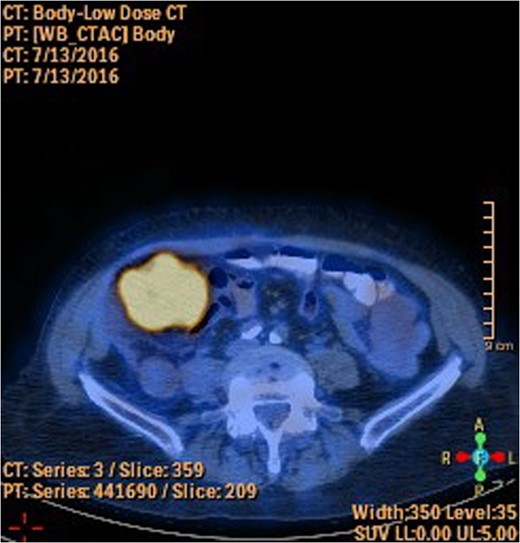
A 78-year-old female presented with worsening abdominal pain over 1 week, with onset of symptoms occurring 3 months prior. No history of recurrent fever or weight loss was elicited. There were no changes in bowel habits and she denied melena or hematochezia. She endorsed a normal colonoscopy three years prior. Of note, she was diagnosed with a biopsy proven squamous cell cancer of her left lower lobe 4 months earlier (Fig. 1). A PET scan performed just three weeks prior to her presentation demonstrated significant FDG uptake in the left lower lobe as well as the cecum, with no convincing evidence of regional nodal disease in the lungs (Figs 2 and 3).
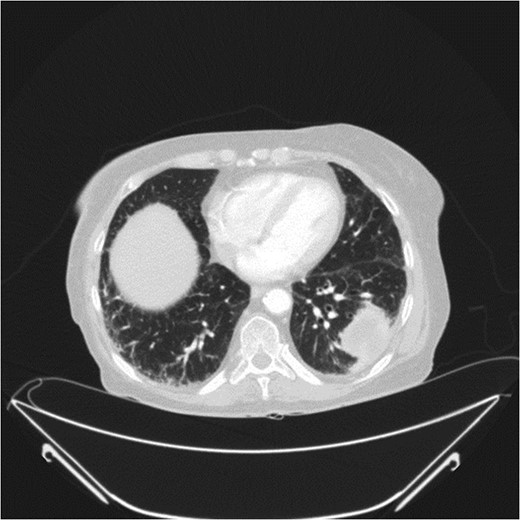
Her past medical history was significant for coronary artery disease with two previous MI’s, COPD, hypertension and type II diabetes mellitus. Past surgeries included an appendectomy, hysterectomy and laparoscopic cholecystectomy. She admits to a 20 pack-year smoking history with minimal alcohol intake.
At presentation her vital signs were stable. Abdominal exam revealed exquisite tenderness in the right lower quadrant, with no evidence of digital clubbing. Blood-work revealed an elevated WBC of 15.6. A CT scan demonstrated a 4.3 cm annular mass within the cecum, suspicious for a primary colonic malignancy, as well as an 8.6 × 8.2 cm2 lobulated gas-containing abscess with extension in to the lateral abdominal wall (Figs 4 and 5).
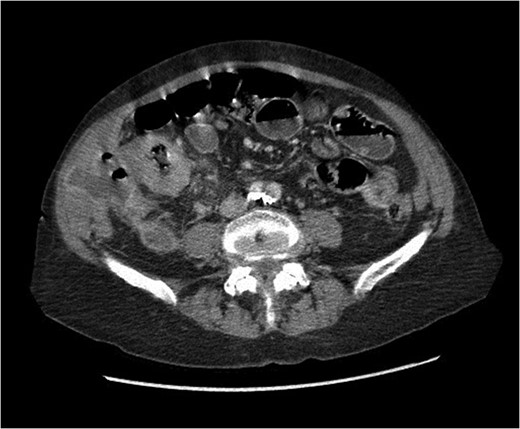
Abdominal CT scan showing annular cecal mass with evidence of perforation.
Urgent exploratory laparotomy revealed a large cecal mass invading the abdominal wall with perforation and localized abscess formation. A right hemi-colectomy was performed with irrigation and washout of the abdomen. Source control was achieved and a primary anastomosis was performed with no diversion. A Jackson-Pratt drain was left in situ. She received 3 days of IV antibiotics with transition to oral antibiotics on POD 4. Her JP drain was removed on POD 3 and she was discharged on POD 5 without complication and with a prescription for oral antibiotics. After extensive histopathology review, the cecal mass was confirmed to be an invasive squamous cell carcinoma of lung origin (Fig. 6). Specimens were sent for immunohistochemical staining. Subsequent investigations included cystoscopy and urine cytology, which were negative for urogenital carcinoma.
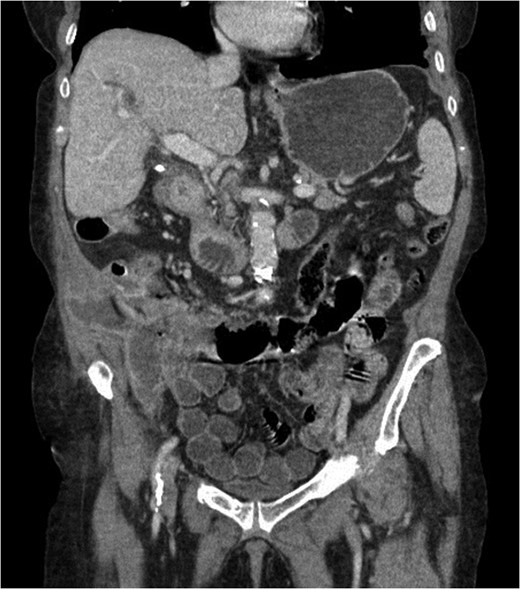
Coronal view of abdominal CT scan showing cecal mass with evidence of perforation.
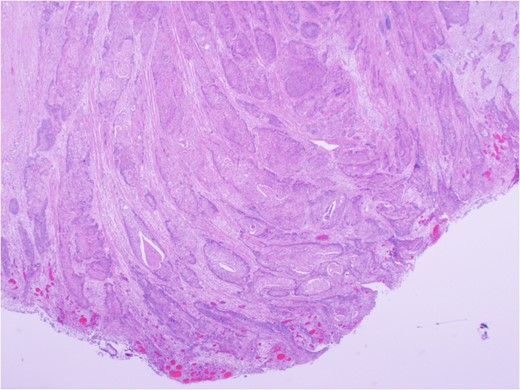
Carcinoma involving colonic serosa (image courtesy Dr Maisoun Abdelbaqi, Department of Pathology, Red Deer Regional Hospital, Alberta, Canada).
DISCUSSION
Historically the esophagus has been thought to be the most common site of lung cancer metastasis within the GI tract, followed by the small bowel [5]. Colonic involvement accounts for <5% of GI metastases [5]. Clinically diagnosed metastatic deposits to the GI tract are far less common than autopsy confirmed lesions [3].
Accurate identification and characterization of GI metastasis is paramount to effective management. Imaging modalities such as CT or MRI are often inadequate for establishing the diagnosis [3]. As a result, accurate diagnosis relies heavily on immunohistochemical staining. A pathology review was performed in this case and should always be considered in this setting. For our patient, p16, TTF1, CK7, CK20, CDX2, PAX5 and NAPSIN A staining were completed on both lung and colonic tissue samples. Both samples were positive only for CK7, and the high degree of correlation allowed for confirmation of the diagnosis.
The importance of establishing the right diagnosis is exemplified in our case. Our patient was referred to a thoracic surgeon for consideration of oncologic resection of her primary lesion. While the presence of a colonic primary certainly influences the decision regarding management, the presence of metastatic disease to the GI tract negates curative resection and portends a poor prognosis. Of note, our patient demonstrated isolated colonic metastasis in the absence of regional nodal disease on PET-CT. The sensitivity of PET-CT in detecting mediastinal disease has been shown to be as low as 78%, likely influenced by a multitude of factors including tumor biology [6]. As such, an area of distant FDG-avid activity should be presumed to represent metastatic disease, even in the absence of regional or mediastinal disease, until proven otherwise.
Given the location of the colonic lesion, squamous cell cancer of GI origin was deemed unlikely. A thorough pathology review by two independent pathologists was performed, which confirmed the suspicion of metastatic disease. Additionally, cystoscopy and urine cytology ruled out the presence of a urogenital primary. Our patient’s disease was deemed incurable and palliative radiation was initiated. Her disease progressed rapidly and she succumbed to her illness 3 months after her presentation.
In summary, GI metastasis from a primary lung carcinoma resulting in large bowel perforation is rare and signals a poor prognosis. Establishing the correct diagnosis is of utmost importance in the setting of potentially curable disease. Thorough histopathology review with appropriate adjuncts should be considered in all cases. Colonoscopy, cystoscopy and urine cytology are also of value.
CONFLICT OF INTEREST STATEMENT
None declared.



