-
PDF
- Split View
-
Views
-
Cite
Cite
Matthew Q Schmidt, Michael Hafertepe, Randall Fortuna, Ibrahim Abdullah, Randy Richardson, Cervical left pulmonary artery, Journal of Surgical Case Reports, Volume 2018, Issue 1, January 2018, rjx252, https://doi.org/10.1093/jscr/rjx252
Close - Share Icon Share
Abstract
We detail what we believe to be the first reported case of a congenital ‘Cervical Left Pulmonary Artery’ in which an aberrant left pulmonary artery courses cranially into the mid-cervical neck before descending back into the thorax to the left pulmonary hilum. Due to the location and course of the artery, we believe that this anomaly is likely due to a developmental error of the sixth pharyngeal arch. Ultimately, the use of a reconstructed 3D computed tomography image provided detailed characterization of the unique anatomical variant, aiding in a successful surgical repair of the defect.
INTRODUCTION
Defects of the aortic arches are rare, potentially lethal cardiac anomalies that form beginning in the third week of development [1]. There are numerous cardiac vascular anomalies attributed to abnormal aortic arch development, including the double aortic arch, right aortic arch, aberrant left or right subclavian artery, anomalous innominate artery or pulmonary artery sling (PAS) [2]. Amongst the larger class, anomalies of the left pulmonary artery (LPA) are particularly rare, with the PAS being the most common.
While mild cases may appear asymptomatic, the majority of neonates with aortic arch abnormalities present with respiratory or upper gastrointestinal symptoms as a result of compression of the trachea and/or esophagus [3]. The use of echocardiography, computed tomographic angiography, magnetic resonance angiography and barium contrast esophagogram can be crucial for diagnosis when a vascular etiology for respiratory or upper digestive symptoms is suspected [1].
CASE REPORT
Our patient is baby boy born at 35 weeks gestation with congenital lobar emphysema, VSD, double outlet right ventricle with the aorta to the right of the pulmonary artery in a side by side relationship. A CT scan performed on Day 11 showed a LPA originating from the posterior aspect of the right pulmonary artery (RPA). 3D computed tomography (3D-CT) reconstruction showed the LPA coursing superiorly into the mid-cervical neck before descending into the left thorax to the left pulmonary hilum (Fig. 1). The anomalous LPA remained anterior to the cervical trachea, and there was evidence of left upper lobe bronchial stenosis. The CT scan also illustrated a double outlet right ventricle, a moderate sized ventricular septal defect, congenital lobar emphysema, abnormal development of the thyroid and increased collateral circulation of the pulmonary arterial system with aortopulmonary collaterals.
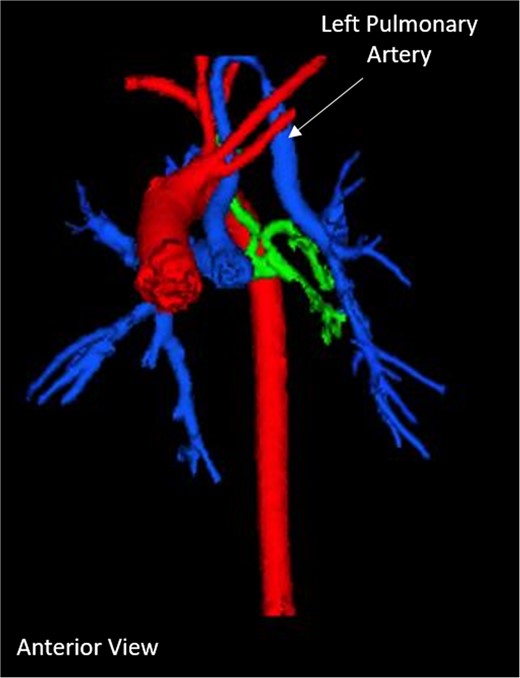
Pre-operative 3D-CT reconstruction showing the left pulmonary artery ascending into the neck.
A left thoracotomy upper left lobe lobectomy and mediastinal cyst removal was performed at 5 weeks of age. Over the next 3 weeks the patient required increased oxygen requirements and repair of his many cardiac defects was recommended. At 8 weeks of age the patient underwent a LPA plasty with supramitral ring resection, RV bundle resections and enlargement of the VSD. The LPA plasty created a spatulated anastomosis between the proximal ascending LPA and the distal descending LPA. A post-surgical CT scan showed successful repositioning of the LPA (Fig. 2). There was a new, post-operative right ventricular outflow tract aneurysm near the junction of the LPA, as well as the persistence of a small VSD.
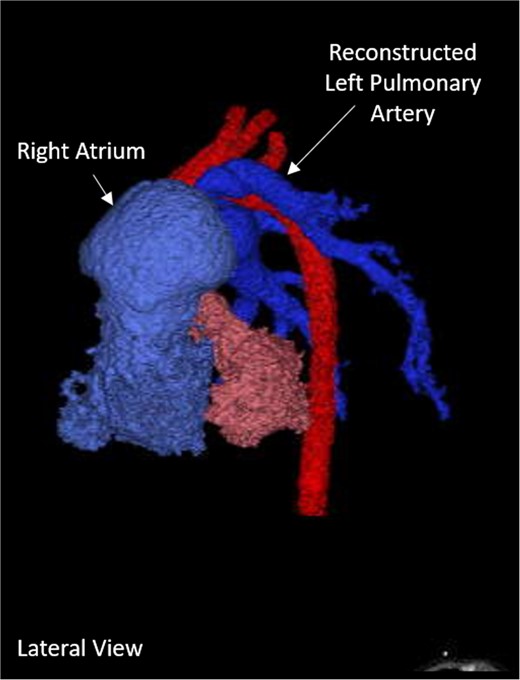
Post-operative 3D-CT reconstruction showing the repaired left pulmonary artery.
Post-operatively, the patient was discharged after adequate recovery. The patient had recurrent respiratory tract infections requiring multiple hospitalizations. Additionally, further echocardiograms during these hospitalizations displayed continued cardiac defects that required further repair at 6 months of age.
DISCUSSION
To our knowledge, this is the first reported case reported of a congenital LPA that extends into the neck. With early signs of bronchial stenosis, it is likely that the patient would have developed severe complications, similar to those seen in other congenital vascular abnormalities. For example, infants with severe tracheal stenosis due to PAS are reported to have mortality rates as high as 90% without surgical correction [2]. Thus, effective surgical repair is of upmost importance in these cases.
Multiple aspects of this case suggest that the etiology is related to a defect in aortic arch development. In normal development, the sixth aortic arch derives from the mesoderm of the sixth pharyngeal arch and forms the pulmonary arteries between the fourth and eighth weeks of development. Other anatomic structures derived from sixth pharyngeal arch mesoderm include the majority of the intrinsic laryngeal muscles and laryngeal cartilage. The recurrent laryngeal nerve (RLN) innervates the laryngeal musculature within the sixth pharyngeal arch, and as the cardiac and aortic structures migrate into the thoracic region, the RLN lengthens along this migratory tract. In the current case, the patient’s LPA ascends to the level of the larynx, the final location of sixth pharyngeal arch structures. After arching, the ‘descending limb’ of the LPA re-enters the thorax in close proximity to the anatomical tract of the left RLN within the left tracheoesophageal groove, the migratory tract of sixth arch structures. Due to these striking relationships, we hypothesize that the cervical LPA is related to a developmental defect in which the LPA failed to fully separate from the other structures of the sixth pharyngeal arch.
There are other less likely mechanisms that warrant consideration. As previously noted, the most common LPA abnormality seen is the PAS, in which the LPA originates from the posterior aspect of the RPA, encircles the right mainstem bronchus, and then passes between the esophagus and trachea as it courses to the left lung [4]. Since our patient’s LPA had a similar origin from the posterior RPA, the two main hypotheses by Jue et al. and Sade et al. [5, 6], which explain the formation of PAS should be considered. These papers theorize that failure of standard LPA development creates the need for an alternative source of blood supply for the left lung; thus, a fairly direct connection forms between the RPA and left lung hilum, which is the resulting aberrant LPA. In the current case, it is unlikely that the anomaly was driven by a need for collateral flow due to the dramatic, indirect course of the LPA into the mid-cervical neck. Additionally, in all reported cases of the PAS, the LPA passes between the trachea and esophagus; in this case, the arch of the cervical LPA remains anterior to the trachea, further differentiating this from the standard PAS and suggesting a unique etiology.
Finally, the use of 3D-CT reconstruction modeling was paramount in detailing the unique and complex anatomy of this infant boy. There are concerns regarding the high levels of radiation exposure from CT in pediatric patients, so this modality should be used sparingly. However, in cases with unique aortic vasculature, a detailed anatomic description is paramount before undertaking interventional or vascular procedures [7]. In this case, the model developed from 3D-CT reconstruction was useful for pre-operative and intra-operative planning, aiding our surgeons in performing a successful repair of the anomaly.
CONFLICT OF INTEREST STATEMENT
None declared.



