-
PDF
- Split View
-
Views
-
Cite
Cite
Miroslav P. Peev, Anne Therese C. Lim, Tianle Zou, Laurence H. Brinckerhoff, Metastatic Epicardial Leiomyoma with uncertain malignant potential, Journal of Surgical Case Reports, Volume 2017, Issue 9, September 2017, rjx179, https://doi.org/10.1093/jscr/rjx179
Close - Share Icon Share
Abstract
A 49-year-old female with history of uterine leiomyoma and intermittent shortness of breath presented to the emergency department with new onset of tachycardia and chest pain. Subsequent cardiac work up revealed hypoechoic mass compressing the right ventricle. Computer tomography guided biopsy for tissue characterization revealed a benign spindle cell tumor. Surgical resection of a large epicardial tumor was undertaken. The histologic examination of the tumor was consistent with Estrogen and Progesterone positive leiomyoma of uncertain malignant potential. To the authors’ knowledge, this is the first case report of a metastasizing epicardial leiomyoma that exhibits an unknown malignant potential. This case brings together common gynecologic disorder with complex thoracic surgery diagnosis and management. Differential diagnosis of cardiac tumors in patients with history of uterine leiomyoma should include metastasizing leiomyoma. The mainstay of therapy is surgical resection with immediate symptom relieve.
INTRODUCTION
Cardiac leiomyoma is an uncommon tumor of the heart previously described as primary neoplasm, benign metastasizing leiomyoma or intracardiac leiomyomatosis. We describe the diagnostic and therapeutic algorithm of a rare epicardial leiomyoma with uncertain malignant potential originating from the right ventricle.
CASE REPORT
A healthy 49-year-old female with history of hysterectomy for uterine leiomyoma was referred to our hospital with tachycardia and acute onset of chest pain. On admission, the patient reported having intermittent shortness of breath and back pain for several years that significantly progressed one-week prior the presentation. The chest pain had both pleuritic and positional components, no heart murmurs or other significant clinical findings were identified. The hematological and biochemical investigations were normal. Subsequent transthoracic echocardiogram showed a round hypoechoic mass within the pericardial space. The tumor was in direct contact with the right ventricle, with no signs of local invasion. There was mild compressive effect with preserved ejection fraction of 50–55% (Figure 1). Computer tomographic (CT) angiography of the chest confirmed an 80 × 60 mm, well-circumcised solid mass of unknown origin. There was no evidence of other primary tumors or acute pulmonary thrombo-embolic disease. CT guided biopsy for tissue characterization revealed a benign spindle cell tumor. The immunohistochemical stains showed tumor cells that are strongly positive for Estrogen and Progesterone receptors (ER and PR) in addition to Smooth Muscle Actin (SMA), Desmin and Caldesmin.
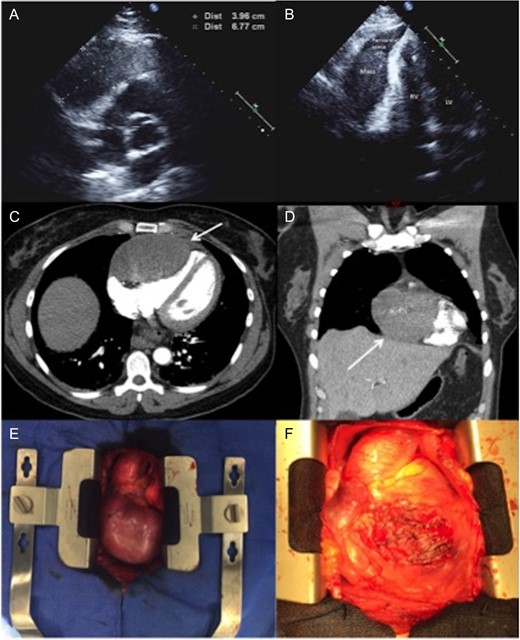
(A and B) Two-dimensional echocardiography. An apical two-chamber view reveals a well-circumscribed, oval hypoechoic mass with maximal diameter of 67 mm. (C and D) Axial and coronal CTA imaging of the tumor showing the direct contact and compression effect to the right ventricle. (E)—Intraoperative pictures demonstrating a large tumor adherent to the right ventricle. (F) The heart after removing of the tumor: almost entire anterior surface of the right ventricle has been involved. LV, left ventricle; RV, right ventricle; CTA, computer tomographic angiography.
The lack of local invasion, the general mass appearance and the pathologic evaluation of the CT guided biopsy suggested a benign nature of the tumor. Additional imaging such as cardiac MRI, PET-CT and TEE (transesophageal echocardiogram) were considered. After multidisciplinary discussion we decided to proceed directly with the operative management of the tumor, taking into account the compression effect and the pronounced symptoms of the patient.
During a subsequent transsternal exploration, a broad-based mass was found to be originating from the visceral pericardium, tightly attached to the underlying right ventricle with no infiltration of the parietal pericardium (Figure 1). The 80 × 65 mm tumor was excised from the right ventricle without the need to place the patient on cardiopulmonary bypass (Figure 2).
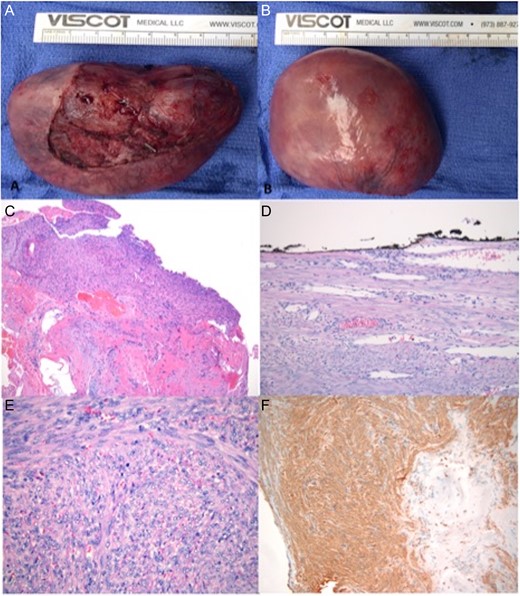
(A and B) Itraoperatively obtained specimen. (C) Myocardial biopsy with ER/PR positive smooth muscle neoplasm composed of intersecting bundles of spindle cells, most resembling leiomyoma of deep soft tissue. Cardiac tissue and perivascular areas of myocardial interstitium adjacent to the tumor were involved. (D) No atypia or necrosis seen. Well circumscribed, mitotically active, and focally abutting the resection margin (inked black). (E) H&E staining of myocardial tumor showing mitotic figures in the tumor. (F) Immunohistochemistry staining positive for SMA.
Macroscopic evaluation of the tumor demonstrated an 80 × 65 × 47 mm (122 gram) well circumscribed, tan-pinked mass, covered by a thin membrane. Histopathologic examination showed smooth muscle neoplasm composed of intersecting bundles of spindle cells, most resembling leiomyoma of deep soft tissue. An immunohistochemical evaluation demonstrated tumor cells that are positive for ER and PR, SMA, desmin and caldesmin; negative for cKit, calretnin, S100, HMB45 and pancytokeratin. The Ki67 was <2% when subtracting the inflammatory cells. The tumor appeared to involve perivascular areas of myocardial interstitium. There was no atypia and no necrosis, however the mitotic count was elevated to 8–11 mitosis in 50 high-power fields with focal involvement of the resection margin. The staining profile in conjunction with the histomorphology was consistent with leiomyoma. The ER/PR staining for nearly 100% of the tumor cells isolated from the heart was a strong sign of female genital origin [1].
The final diagnosis was smooth muscle neoplasm likely corresponding to metastatic epicardial leiomyoma of uncertain malignant potential. The patient’s postoperative course and 6 months follow up were uneventful.
DISCUSSION
Primary cardiac tumors are extremely uncommon and have a reported autopsy frequency of only 0.001–0.03% [2]. Seventy-five percentage of these are benign with almost half being myxomas. Other benign tumors such as lipoma, papillary fibroelastoma and rhabdomyoma have also been described. The remaining 25% of cardiac neoplasms are found to have malignant potential with the vast majority (95%) being sarcomas such as angiosarcoma [2].
Cardiac tumors are associated with wide variety of symptoms depending on their location and size. The most commonly presenting symptoms are pain, shortness of breath, obstructive or heart failure symptoms, arrhythmia or embolization. The initial diagnosis is usually made by transthoracic echocardiography, while magnetic resonance imaging and computer tomography have also been used for further characterization of those tumors. Smooth muscle tumors found in the heart have also been described and are divided into three main categories: primary cardiac leiomyoma or leiomyosarcoma (if malignant), intravenous smooth muscle tumors with intracardiac extension and benign metastasizing leiomyoma [3].
Benign metastasizing leiomyoma is a rare complication of myometrial leiomyoma that presents after hysterectomy with lungs and pelvis being the most frequent sites of occurrence [1]. It has been speculated to be due to tumor embolism through venous channels at the time of previous hysterectomy. The tumor spreads through the uterine veins, reaching into the inferior vena cava and the right heart chambers.
While the vast majority of reported cardiac benign metastasizing leiomyomas are found within the heart, our patient presented with an extremely rare form of epicardial metastasizing leiomyoma involving the right ventricle.
To the best of our knowledge, this is the first case report of a metastasizing epicardial leiomyoma that exhibits an unknown malignant potential. Leiomyomas of deep soft tissue usually display no atypia and no mitotic activity. In our patient, the smooth muscle neoplasm had increased mitotic rate, in addition to focal involvement of the underlying cardiac tissue. The diffuse nuclear pattern of ER and PR expression among the tumor cells strongly supported a female genital origin.
After the final diagnosis was established a second histopathological opinion was obtained in order to confirm the metastizing nature of the tumor. A direct pathology comparison of the uterine and the cardiac tumors was considered, attempted but not possible due to the geographical location of the initial uterine surgery and the accessibility of the histopathology results.
The best designation for this lesion is metastasizing epicardial leiomyoma with uncertain malignant potential. Based on the pathologic features, close follow-up is recommended in order to detect potential early tumor recurrence.
CONFLICT OF INTEREST STATEMENT
None declared.



