-
PDF
- Split View
-
Views
-
Cite
Cite
Silvia Pereira, Wilson Malta, António Canha, José Polónia, Vacuum-assisted closure therapy after resection of giant basal cell carcinoma of the scalp, Journal of Surgical Case Reports, Volume 2017, Issue 7, July 2017, rjx122, https://doi.org/10.1093/jscr/rjx122
Close - Share Icon Share
Abstract
Management of complicated wounds is a challenge in head and neck reconstruction. Although the negative pressure wound therapy or wound vacuum-assisted closure has been widely used in complicated wounds and shows promising results, its application in the head and neck region after reconstruction for the head and neck cancer is rarely presented. A 77-year-old woman underwent a radical resection of an extensive basal cell carcinoma of the scalp and forehead involving the periosteum, where classic reconstruction was difficult, but successfully treated with negative pressure wound therapy. Negative pressure wound therapy is an efficacious tool in cases of complex and extensive defects, when we expect immediate reconstruction with poor results, as would be probable with this scalp lesion.
INTRODUCTION
Basal cell carcinoma (BCC) is the most common neoplastic tumor of the skin, most frequently located in the head and neck area [1].
Scalp wounds may be classified with respect to treatment into superficial wounds, deep scalp wounds and deep scalp wounds with exposed calvarial bone. Defects involving damage to the periosteum, with exposure of the denuded cranial calotte are a rare, but enormous challenge. While smaller periosteal defects may be treated with a free or pedicled flap, larger areas are a major challenge, because of specific features of this anatomical site. New developments in grafting, artificial skin substitutes and vacuum-assisted closure, as well as other advances, enabled new treatment options [2, 3]. Although the negative pressure wound therapy (NPWT) has been widely used in complicated wounds showing promising and consistent results, its application in the head and neck region after reconstruction or as a temporary measure when immediate reconstruction would not be feasible [4, 5]. This might be due to difficulties to place air-tight dressings at the head or neck, especially in the presence of hair-bearing skin in this region. There are few published studies regarding the use of negative pressure therapy in wounds located on the head. We present a case with an extensive BCC of the scalp and forehead involving the periosteum, successfully treated with aid of NPWT. Such complex defects, are a challenge by the size, location and type of treatment, have no data on the literature with the features we describe.
CASE REPORT
A 77-year-old woman presented with a 3-year history of an extensive expanding BCC involving the scalp and the forehead, with initial refusal of treatment. Histological investigation with incisional biopsy, confirmed a mixed BBC, nodular-cystic, reticular and infiltrating type (Fig. 1).
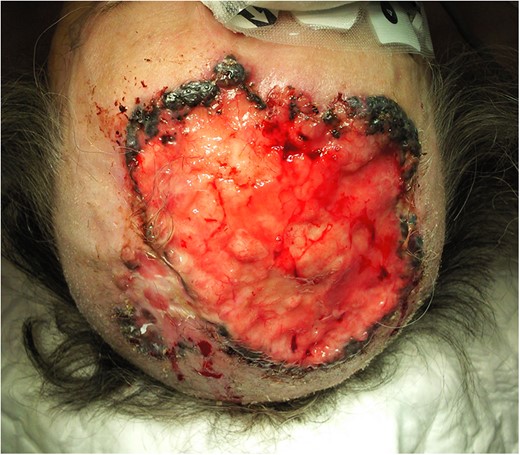
Extensive BCC involving the scalp and the forehead, preoperative finding.
Radical resection was then proposed. In the following surgical intervention, the scalp was resected with macroscopically negative margins. The defect measured 14 cm × 12 cm and the neoplastic tumor measured 12,5 cm × 11,3 cm. Minimal tumor-free margins were 7 mm (Fig. 2A–C).
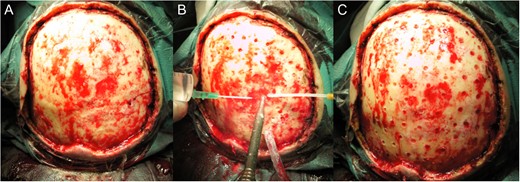
Intraoperative view of the resection defect. (A) without periostium, (B) and (C) during and after drilling.
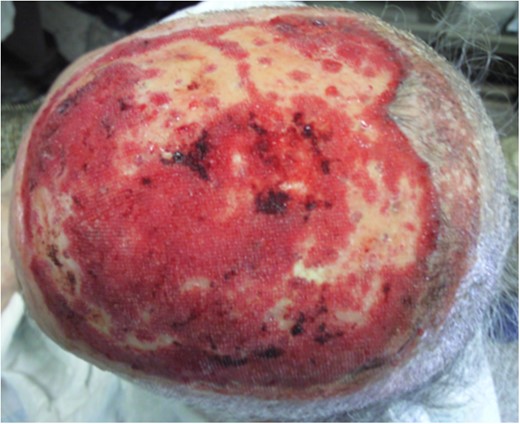
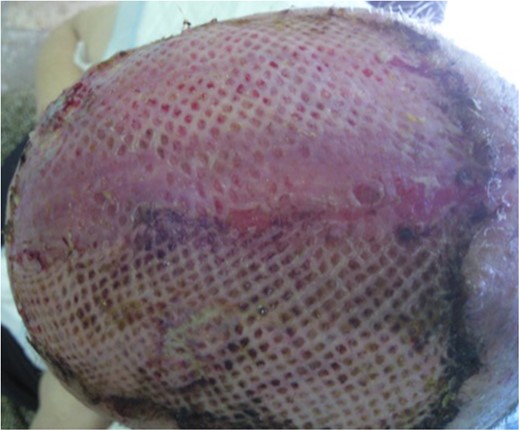
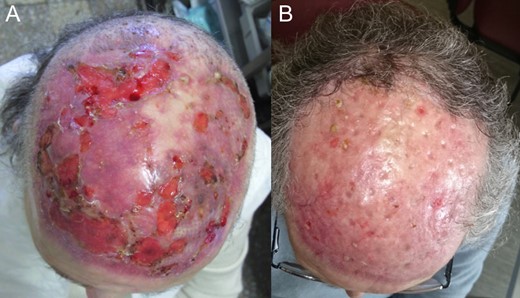
One week after surgery with classic dressing, it presented with necrosis and no proliferation of scar tissue (Fig. 3). We started NPWT at that time (Fig. 3B–E). Following 6 weeks with NPWT, we achieved conditions for split skin graft (SSG) with good final results (Figs 4, 5 and 6A and B). There was no place for radiation therapy in this case.
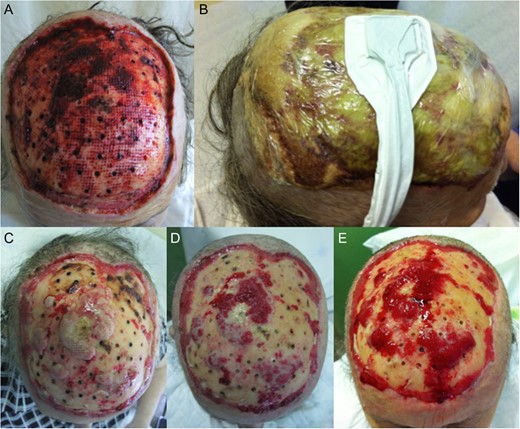
(A) One week after surgery, with classic dressing with necrosis and no proliferation of tissue. (B) Starting of negative pressure wound therapy at that time. (C) One week after, (D) 2 weeks after and (E) 3 weeks after.
DISCUSSION
BCC are the most common cutaneous neoplasms, the incidence is increasing, and is usually observed in older patients, especially in those extensively exposed to ultraviolet radiation during their lives. The most typical site of BCC is uncovered skin directly exposed to the sun. Thus, BCC is often observed in head and neck areas, and nearly two-thirds of BCC occur in the head [1, 6]. Some BCC display an aggressive growth pattern and rapidly reach a large size. Only a few cases of BCC of the scalp with radiological documentation of bone erosion and invasion of the subjacent dura mater have been reported [1].
BCC is potentially curable, but local recurrence rate is as high as 50% if the tumor has been treated inadequately in the first instance. When left untreated or inadequately treated, a BCC can reach enormous size destroying the whole face penetrating deeply through subcutaneous tissue, even bone [1].
To achieve a favorable outcome in BCC treatment, it is important to recognize the histological subtypes, identify the anatomic locations that can increase the risk of spread, and understand the limitations of all available treatment modalities. Defects after surgical excision may be repaired by adequate local flaps. Scalp reconstruction for large defects following tumor resection is a challenging problem for many reasons, including the excessive area of these defects, commonly exceeding 50 cm2, the relatively limited elasticity of scalp tissues, the relatively poor vascularization (tumors are commonly locally advanced, recurrent after previous resection or subjected to previous irradiation), and the distance of the scalp from donor sites. In addition, tumor resection may be a complex procedure when there is calvarial bone involvement [4].
The management of complicated wounds by NPWT was first introduced in 1980 in Russia, and the first commercial equipment used in 1993, by Argenta and Morykwas [7, 8], and applied thereafter in many specialities, including plastic surgery, general surgery and orthopedics [9]. NPWT was rarely presented as an option after head and neck cancer surgery, specially in extensive cases [5]. This might be due to the anatomic complexity of the head and neck area that makes it sometimes difficult to apply negative pressure in large defects [10]. In our case, the extensive BCC of the scalp and forehead involving the periosteum, classic dressing presented with necrosis and no proliferation tissue, but was successfully treated with NPWT, because it provided a safe wound environment applied directly onto the periostium [4]. In this context, NPWT allows the formation of a protective, optimized wound environment in preparation for late reconstruction [4].
CONFLICT OF INTEREST STATEMENT
None declared.



