-
PDF
- Split View
-
Views
-
Cite
Cite
Luen Shaun Chew, Xinguang Julian Han, Kheng Kooi Tan, Manish Mahadeorao Bundele, Hemangiopericytoma of the thoracic spine: a case report, Journal of Surgical Case Reports, Volume 2017, Issue 7, July 2017, rjx121, https://doi.org/10.1093/jscr/rjx121
Close - Share Icon Share
Abstract
Hemangiopericytoma (HPC) has been described to be aggressive and potentially a malignant tumour. We report a rare case of a 63-year-old Chinese male who presented with primary intradural extramedullary HPC of the thoracic spine. The main presenting complaint was gradual progression of back pain, associated with paraparesis and sensory deficit of lower limbs. He had MRI thoracolumbar with contrast which showed T9 lesion compressing on spinal cord and oedema, he was then operated upon and histopathology report confirmed a thoracic spine HPC. A T8/9 laminectomy and excision of intradural extramedullary lesion was performed, tumour section was sent for frozen section study, and more tissue was sent for paraffin studies and additional immunohistochemical staining. Surgical resection is most commonly performed, radiotherapy remains debatable. In this report, we discussed another rare case of primary spinal HPC to be added into the literature.
INTRODUCTION
Hemangiopericytoma (HPC) is a rare tumour that arises from pericapillary cells or pericytes of Zimmermann as first described by Stout and Murray [1]. In the current literature, only around 80 cases of spinal HPC have been reported and more commonly they present as intradural-extramedullary tumours [2]. It is a highly vascular neoplasm which could occur anywhere in the body where capillaries are present. They are most commonly found in musculoskeletal system and skin [1, 3], rare in the central nervous system as they only account for <1% of all central nervous tumours [4]. These tumours were first considered as angioblastic variants of meningiomas but the World Health Organisation (WHO) recognised these tumours as distinct clinicopathological entities in 1993 [5]. In the perspective of central nervous system involvement, this tumour more commonly presents in the cranium, very rarely does it manifest in the spine; most often being intradural and extramedullary [6]. Diagnosis of HPC is via histopathology, gold standard of treatment is complete surgical resection, benefits of radiotherapy remains controversial [7].
We present a rare case of a non-metastatic intradural extramedullary thoracic spine HPC which was treated in our institution.
CASE REPORT
A 63-year-old Chinese male presented to emergency department with history of back pain for 8 months, worsened over the last 5 days prior to admission. He also complained of associated bilateral lower limb numbness and weakness. He had tried several sessions of physiotherapy but did not help with the back pain. He denied recent falls or trauma to the back. He had no issues passing urine and no changes to bowel habit. These symptoms had affected his mobility so much so he had to resort to walking stick for mobility assistance. On physical examination, his gait was unsteady, back pain exacerbated on movement. There was paravertebral and spinal tenderness at level T5–T10, decreased sensation on bilateral lower limbs. Motor examination revealed paraparesis of bilateral lower limbs of grade 2/5 Medical Research Council (MRC). There was no saddle anaesthesia and anal tone was intact on per rectal examination. Upper limb neurological examination was unremarkable. Bloods were unremarkable. Erythrocyte sedimentation rate (ESR), inflammatory markers (white cell count, and C-reactive protein [CRP]) were not raised. Urea and electrolytes were within normal ranges.
X-ray thoracolumbar spine anterior-posterior and lateral views were performed and showed slight loss of the T6 anterior vertebral height and sclerosis of the T5/T6 endplates. Mild narrowing of the T5/T6 intervertebral disc space was also seen and there were no evidence of fractures or spondylolisthesis. Magnetic resonance imaging (MRI) of the spine with contrast was then requested, showing 1.6 × 1 × 1.2 cm3 enhancing lesion in the spinal cord at level T9, mostly extramedullary intradural location compressing the thoracic cord with extensive cord oedema from T1 down to conus (Fig. 1). It was also suggestive of intramedullary invasion into the spinal cord, from the extramedullary enhancing mass lesion.
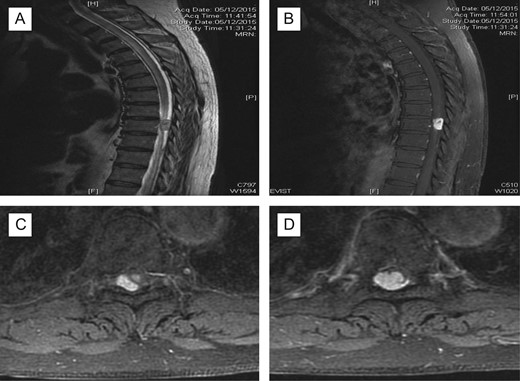
Pre-op MRI scan. Enhancing intraspinal lesion, most likely extramedullary intradural in location, at the level of T9 causing spinal cord compression associated with extensive cord oedema; (A) T2 sagittal; (B) with contrast, sagittal; (C, D) axial with contrast at T9 level; suggestive of intradural extramedullary (C) extending intramedullary (D).
A decision was taken to operate the patient in view of cord compression, progressive neurological deterioration and for histopathology confirmation of the diagnosis. A T8/T9 laminectomy and excision of intradural extramedullary lesion was performed. The tumour was grossly reddish in colour; located purely extramedullary and near total excision was performed. Post-operatively, patient had MRI cervical spine, MRI brain with contrast and CT thorax, abdomen, pelvis for further evaluation of the disease; scans were unremarkable with no evidence of primary source/ metastatic lesions. The pain improved significantly, lower limb power improved and patient was subsequently transferred to rehabilitation centre to optimise recovery to achieve mobility independence. Twelve-month follow-up at clinic revealed significant improvement in back pain and in bilateral lower limb power, with no evidence of recurrence. Repeat MRI scans showed interval improvement of the disease (Fig. 2). Patient remained in rehabilitation unit and was discharged home after 6 months.
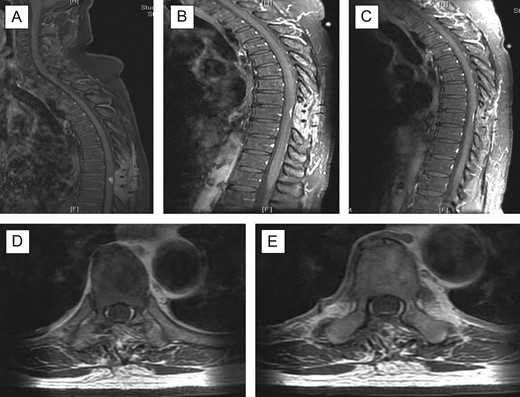
Post-operative follow-up MRI scan. There is residual focus of dural thickening and enhancement at the level T9 which demonstrated further reduction in prominence (A–C); there is resolution of the cord oedema and no new enhancing lesion is seen in the spinal canal; (A) sagittal with contrast 3 months’ follow-up; (B) 6 months’ follow-up; (C) 11 months’ follow-up; (D + E) axial views with contrast 11 months’ follow-up at T9 level.
The histopathology sections (Fig. 3) showed hypercellular areas containing a proliferation of spindle cells arranged in a vague storiform-like pattern, present around numerous small calibre and ectatic blood vessels. The cells had bland ovoid to round nuclei, eosinophilic cytoplasm with indistinct cell borders. Looser myxoid stroma was seen in the hypocellular areas. There were no mitoses, necrosis or areas of marked hypercellularity. Reticulin highlighted the vascular pattern. On immunohistochemical staining, the spindle cells were diffusely positive for vimentin and focally positive for Factor 13a. CD34 highlighted the vasculature and showed scattered positive intervening cells. They were negative for EMA and S100 protein. A diagnosis of HPC (WHO Grade II) was made.
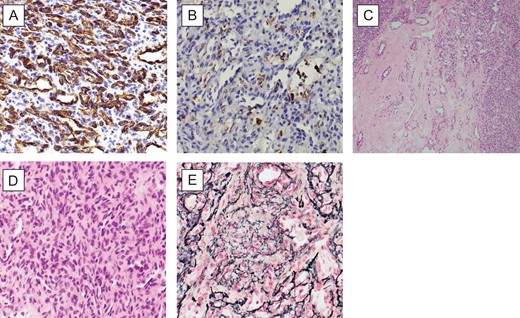
(A) (CD34X40): patchy CD34 immunoreactivity in tumour cells and endothelial cells; (B) (FXIIIAX40): focal FXIIIa positivity in tumour cells; (C) (HEX10): highly vascular spindle cell tumour with numerous slit like and staghorn vascular spaces, featuring hypocellular and cellular areas; (D) (HEX40): closely packed randomly oriented bland spindle cells; and (E) (RETICULINX40): well-developed network of reticulin fibres surrounding individual cells.
DISCUSSION
HPC was once regarded as a different type of meningioma typically because it resembles the latter in clinical and radiological presentation, and because of their similar surgical management [8]. HPC may occur anywhere in the human body but most commonly present in extremities, pelvis, retroperitoneum, head and neck areas [9]. Rarely are they reported in the central nervous system, accounting for around 2% of primary meningeal tumours and <1% of primary central nervous system tumours [10]. HPC is a rare disease particularly spinal HPC. In the current literature, only around 80 cases of spinal HPC have been reported and more commonly they present as intradural-extramedullary tumours [2].
It has non-specific imaging characteristics and clinical manifestations, making it difficult to be correctly diagnosed on the basis of these presentations. It requires combination of radiographic and histological studies for final diagnosis. Differential diagnoses of spinal HPC include meningioma, schwannoma, neurofibroma and neuroblastoma [8].
In our case, MRI spine with contrast was initially suggestive of an intramedullary involvement from an intradural extramedullary lesion. However, intraoperatively, the lesion appeared grossly extramedullary; subtotal excision of the lesion was performed as it was adhered very closely to the spinal cord. Pre-operative embolization was not done; we did not start chemotherapy too as there were no evidence of metastases. Following 6 months from operation, we offered radiotherapy as adjuvant for patient however he declined it because his symptoms were improving following rehabilitation therapy. His follow-up scans also showed further reduction of cord oedema and residual of the lesion. In view of these, he opted for conservative management with a 6-month follow-up scan. The latest scan (11 months post-operative) showed interval regression of cord oedema and no new enhancing lesions were seen (Fig. 2c). There was also no evidence of recurrence and metastasis of disease following 12 months. Gold standard for diagnosis is histological studies, mainstay treatment for HPC would be surgical resection with radiotherapy if residual disease present post-operatively or chemotherapy in event of metastasis. Close and regular long term follow-up (at least 6 monthly) is recommended for HPC in view of its malignant and recurrence potential [11].
FUNDING
None.
CONFLICT OF INTEREST STATEMENT
None declared.



