-
PDF
- Split View
-
Views
-
Cite
Cite
Gary Sharp, David Yeo, Cherry Koh, Presentation of secondary parasitic infection 37 years after primary infection, Journal of Surgical Case Reports, Volume 2017, Issue 6, June 2017, rjx103, https://doi.org/10.1093/jscr/rjx103
Close - Share Icon Share
Abstract
Echinococcus granulosus (EG) is a neglected pathology that causes cystic echinococcosis and primarily affects the liver and lung. EG infects ~6 million worldwide and mortality is quoted as 2–4% per 100 000 inhabitants. The increase in human traffic from endemic regions demands clinician’s awareness. Dogs are the most common definitive host for the EG tapeworm. Human infection requires ingestion of fecal parasitic eggs. Primary infection causes cysts to appear in affected organs, rupture of which leads to secondary infection. Ultrasound remains the mainstay of diagnosis. Treatment can be either; chemotherapeutic, radiological, surgical or a combination depending on the organ affected.
INTRODUCTION
As many as nine genotypes of echinococcus exist [1], however, two species, namely Echinococcus multiocularis and Echinococcus granulosus(EG), cause the majority of human parasitic infection. EG infection causes cystic echinococcosis disease [2–4] (AKA hdatid disease). The following discussion will focus on EG and subsequent cystic echinococcosis only.
EG has 10 genotypes (G1–10) not all of whom are infective to humans [2]. G1 is responsible for most human cystic echinococcosis (88.44%) with its origin being hypothesized to the Middle East [2]. Cystic echinococcosis disease is a neglected pathology [1, 5] that primarily affects the liver [2] and lung [1, 3–6]. Our patient presented with secondary infection involving pelvic viscera and peritoneum. Infection results in major morbidity [1] and remains a huge health issue [6] due to its chronicity [7], life threatening sequelae [1] and possible mortality if untreated [3].
CASE REPORT
A 59-year-old Bosnian male presented with abdominal discomfort. A 20 × 10 cm2 suprapubic mass was palpable. An abdominal ultrasound scan (US) revealed a large complex pelvic mass, subsequent abdominal and pelvic computed tomography (CT) revealed multiple complex peritoneal cysts suggestive of hydatid disease (Fig. 1).
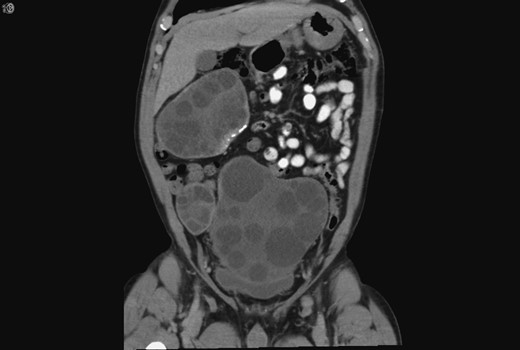
Coronal CT showing the hydatid cysts with daughter cysts within the larger structures.
As a child he reported frequent contact with livestock and dogs. Upon military conscription, aged 17, investigations revealed a pulmonary mass, resection of which confirmed echinococcosis. Five years later, he was diagnosed with splenic hydatid disease culminating in splenectomy. The multiple large cysts and daughter cysts now seen on his CT were presumed secondary to spillage and peritoneal seeding of hydatid cysts during his splenectomy 37 years prior.
He commenced Albendazole for 9 weeks prior to planned surgical resection. During laparotomy, one pelvic and four abdominal cystic structures were discovered. The pelvic cyst was sizeable and densely adherent to the bladder anteriorly and rectum posteriorly. As the cyst entered the true pelvis dissection proved problematic, as such the cyst was surrounded by hypertonic saline soaked packs to inhibit contents spillage. Four stay sutures were placed into the cyst wall and a cystostomy performed. The contents was judiciously evacuated (Fig. 2). Once decompressed, the cyst was sutured closed and resected (Fig. 3).
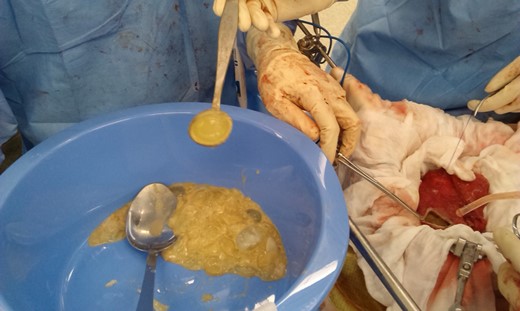
Intraoperative photograph showing the cystotomy and contents being removed. Daughter cysts are shown in the bowl (clear, jelly like structures).
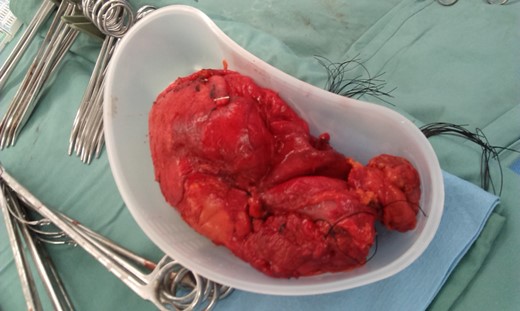
The pelvic cyst once removed minus hydatid fluid and daughter cysts.
Histology showed hydatid cysts and a diagnosis of EG was postulated. He completed a 7-day post-operative course of Praziquantel and 6 months of Albendazole. One year post-surgery repeat CT and MRI abdomen and pelvis show no recurrence.
DISCUSSION
Cystic echinococcosis affects poorer communities [1] based around livestock [4] with close contact to dogs [2]. Accurate prevalence and incidence statistics are lacking due to the disease affecting poorer, rural communities without access to medical care [2]. However, some studies suggest EG infects ~6 million worldwide [7] with prevalence appearing to increase with age [3]. Some indicate females are more prone to infection [2] due to tending livestock and meal preparation [3]. However, hospital based studies indicate the opposite, and found young middle aged males were most affected [3]. Mortality is quoted as 2–4% per 100 000 inhabitants [2].
EG is a tapeworm that belongs to the Taeniidae family [3] and is a zoonotic disease [8]. Dogs are the most common definitive host for EG which attaches itself to small bowel mucosa [3]. Upon defecation parasitic eggs are excreted by infected dogs. Inadvertent livestock consumption of infected pasture results in an intermediate host [9]. Human infection requires ingestion of faecal parasitic eggs [1, 3]. This may occur through physical contact with infected domestic dogs [10], livestock [2] or via contaminated water or vegetables [9]. Humans are opportunistic intermediate hosts only [3] and are unable to spread the parasitic infection. Upon consumption parasitic eggs are absorbed via intestinal mucosa, enter the portal circulation and/or lymphatics [1] and finally reside in viscera, most commonly the liver and lung [6]. Secondary visceral infection occurs due to intraperitoneal cyst rupture or systemic dissemination [6].
Infection may be asymptomatic for many years [8]. The most common presenting complaints with intra-abdominal cystic echinococcosis are pain, fever, jaundice, nausea, vomiting and weight loss [2, 3]. Symptoms may be due to visceral compression, secondary cyst infection or rupture [6]. Intra-abdominal cyst rupture results in release of highly immunogenic hydatid fluid with potential peritoneal seeding [6] and anaphylaxis which is associated with greater mortality [10]. Pelvic cysts present similarly with symptoms due to pelvic organ compression [6].
Serological diagnosis attempts to detect specific IgG antibodies which are produced once hydatid fluid leaches from the cyst into plasma [4]. Unfortunately, serological diagnosis has low sensitivity and specificity [4]. Imaging is the mainstay of diagnosis [4] and abdominal US remains the gold standard [10] with a sensitivity of 90–95% [8]. Cysts are usually anechoic with a double echogenic wall and may show the ‘snowstorm sign’ due to disturbance of hydatid sand within the cyst [6]. The presence of daughter cysts, smaller cysts within the larger mother cyst, may also be present [8] with calcification being a later sign [6].
CT shows well defined cyst walls and possible daughter cysts which are usually peripherally placed [6]. CT has a sensitivity of 95–100% [8] and highlights calcified and pelvic cysts well [6]. Magnetic resonance imaging accurately shows cysts structure and may be superior for post-operative surveillance [6].
Treatment is complex [4] and influenced by location, size and number of cysts coupled with available resources [1, 5]. Benzimidazoles, such as Albendazole remain the most clinically used chemotherapy [1] which is continued for weeks to months [10]. Praziquantel can be used in conjunction with Benzimidazoles [10].
Radiological management involves two main interventions; percutaneous puncture, aspiration, instillation and reaspiration (PAIR) and/or permanent catheterization for larger cysts. PAIR is carried out by aspirating at least one-third of the cysts contents, instillation of an ethanol or hypertonic saline which is left in situ for >5 min, then aspirated [6]. PAIR inactivates the metabolically active cyst layer and is recommended for treatment of cysts of >5 cm [5]. Benefits of PAIR include reduced cost, morbidity, mortality and duration of hospital stay when compared to surgery, but is only indicated for uncomplicated, unilocular cysts without communication to other viscera or ducts [5]. Risks involve anaphylaxis and infection of the cyst [5]. Cysts larger than 10 cm may be permanently catheterized for continued drainage [5].
Surgical intervention used to be the mainstay [1] especially in larger cysts [8], but PAIR and chemotherapy have reduced this need [5]. However, circumstances still exist where surgery is the most beneficial option, e.g. cysts containing daughter cysts not amenable to PAIR [5]. Surgery is used in conjunction with chemotherapy [1] to reduce the risk of anaphylaxis [10]. Follow up for many years [5] with MRI imaging [6] and serological testing is paramount due to potential relapse [4].
CONFLICT OF INTEREST STATEMENT
None declared.



